Related Research Articles
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient.
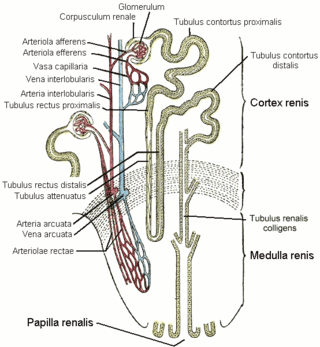
The distal convoluted tubule (DCT) is a portion of kidney nephron between the loop of Henle and the collecting tubule.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

In the kidney, the loop of Henle is the portion of a nephron that leads from the proximal convoluted tubule to the distal convoluted tubule. Named after its discoverer, the German anatomist Friedrich Gustav Jakob Henle, the loop of Henle's main function is to create a concentration gradient in the medulla of the kidney.

Loop diuretics are diuretics that act on the Na-K-Cl cotransporter along the thick ascending limb of the loop of Henle in nephrons of the kidneys. They are primarily used in medicine to treat hypertension and edema often due to congestive heart failure or chronic kidney disease. While thiazide diuretics are more effective in patients with normal kidney function, loop diuretics are more effective in patients with impaired kidney function.

Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They enable coupled or cotransport and include antiporters and symporters. In general, cotransporters consist of two out of the three classes of integral membrane proteins known as transporters that move molecules and ions across biomembranes. Uniporters are also transporters but move only one type of molecule down its concentration gradient and are not classified as cotransporters.
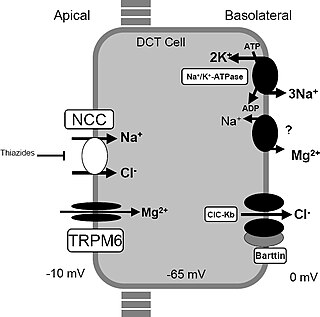
Gitelman syndrome (GS) is an autosomal recessive kidney tubule disorder characterized by low blood levels of potassium and magnesium, decreased excretion of calcium in the urine, and elevated blood pH. The disorder is caused by disease-causing variants in both alleles of the SLC12A3 gene. The SLC12A3 gene encodes the thiazide-sensitive sodium-chloride cotransporter, which can be found in the distal convoluted tubule of the kidney. The distal convoluted tubule of the kidney plays an important homeostatic role in sodium and chloride absorption as well as of the reabsorption of magnesium and calcium.

Bartter syndrome (BS) is a rare inherited disease characterised by a defect in the thick ascending limb of the loop of Henle, which results in low potassium levels (hypokalemia), increased blood pH (alkalosis), and normal to low blood pressure. There are two types of Bartter syndrome: neonatal and classic. A closely associated disorder, Gitelman syndrome, is milder than both subtypes of Bartter syndrome.
In the physiology of the kidney, tubuloglomerular feedback (TGF) is a feedback system inside the kidneys. Within each nephron, information from the renal tubules is signaled to the glomerulus. Tubuloglomerular feedback is one of several mechanisms the kidney uses to regulate glomerular filtration rate (GFR). It involves the concept of purinergic signaling, in which an increased distal tubular sodium chloride concentration causes a basolateral release of adenosine from the macula densa cells. This initiates a cascade of events that ultimately brings GFR to an appropriate level.
Sodium-dependent glucose cotransporters are a family of glucose transporter found in the intestinal mucosa (enterocytes) of the small intestine (SGLT1) and the proximal tubule of the nephron. They contribute to renal glucose reabsorption. In the kidneys, 100% of the filtered glucose in the glomerulus has to be reabsorbed along the nephron. If the plasma glucose concentration is too high (hyperglycemia), glucose passes into the urine (glucosuria) because SGLT are saturated with the filtered glucose.

The sodium-chloride symporter (also known as Na+-Cl− cotransporter, NCC or NCCT, or as the thiazide-sensitive Na+-Cl− cotransporter or TSC) is a cotransporter in the kidney which has the function of reabsorbing sodium and chloride ions from the tubular fluid into the cells of the distal convoluted tubule of the nephron. It is a member of the SLC12 cotransporter family of electroneutral cation-coupled chloride cotransporters. In humans, it is encoded by the SLC12A3 gene (solute carrier family 12 member 3) located in 16q13.
In molecular biology, the electroneutral cation-Cl family of proteins are a family of solute carrier proteins. This family includes the products of the Human genes: SLC12A1, SLC12A1, SLC12A2, SLC12A3, SLC12A4, SLC12A5, SLC12A6, SLC12A7, SLC12A8 and SLC12A9.

Within the nephron of the kidney, the ascending limb of the loop of Henle is a segment of the heterogenous loop of Henle downstream of the descending limb, after the sharp bend of the loop. This part of the renal tubule is divided into a thin and thick ascending limb; the thick portion is also known as the distal straight tubule, in contrast with the distal convoluted tubule downstream.

WNK , also known as WNK1, is an enzyme that is encoded by the WNK1 gene. WNK1 is serine-threonine protein kinase and part of the "with no lysine/K" kinase WNK family. The predominant role of WNK1 is the regulation of cation-Cl− cotransporters (CCCs) such as the sodium chloride cotransporter (NCC), basolateral Na-K-Cl symporter (NKCC1), and potassium chloride cotransporter (KCC1) located within the kidney. CCCs mediate ion homeostasis and modulate blood pressure by transporting ions in and out of the cell. WNK1 mutations as a result have been implicated in blood pressure disorders/diseases; a prime example being familial hyperkalemic hypertension (FHHt).

Potassium-chloride transporter member 5 is a neuron-specific chloride potassium symporter responsible for establishing the chloride ion gradient in neurons through the maintenance of low intracellular chloride concentrations. It is a critical mediator of synaptic inhibition, cellular protection against excitotoxicity and may also act as a modulator of neuroplasticity. Potassium-chloride transporter member 5 is also known by the names: KCC2 for its ionic substrates, and SLC12A5 for its genetic origin from the SLC12A5 gene in humans.

Potassium-chloride transporter, member 4 is a chloride potassium symporter protein. It is encoded by the gene SLC12A4.
The chloride potassium symporter is a membrane transport protein of the solute carrier family 12 that is present in the S3-segment of the renal proximal tubule and in the neuron. It functions in renal chloride reabsorption to transport chloride across the basolateral membrane. Chloride potassium symporter can lower intracellular chloride concentrations below the electrochemical equilibrium potential.
Electroneutral sodium bicarbonate exchanger 1 is a protein that in humans is encoded by the SLC4A8 gene.

Solute carrier family 12 member 6 is a protein that in humans is encoded by the SLC12A6 gene.
The cation-chloride cotransporter (CCC) family is part of the APC superfamily of secondary carriers. Members of the CCC family are found in animals, plants, fungi and bacteria. Most characterized CCC family proteins are from higher eukaryotes, but one has been partially characterized from Nicotiana tabacum, and homologous ORFs have been sequenced from Caenorhabditis elegans (worm), Saccharomyces cerevisiae (yeast) and Synechococcus sp.. The latter proteins are of unknown function. These proteins show sequence similarity to members of the APC family. CCC family proteins are usually large, and possess 12 putative transmembrane spanners (TMSs) flanked by large N-terminal and C-terminal hydrophilic domains.
References
- ↑ Haas M (October 1994). "The Na-K-Cl cotransporters". Am. J. Physiol. 267 (4 Pt 1): C869–85. doi:10.1152/ajpcell.1994.267.4.C869. PMID 7943281. S2CID 22680398.
- ↑ Hebert, SC; Mount, DB; Gamba, G (February 2004). "Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family". Pflügers Archiv: European Journal of Physiology. 447 (5): 580–593. doi:10.1007/s00424-003-1066-3. PMID 12739168. S2CID 21998913.
- ↑ Russell, J. M. (January 2000). "Sodium-potassium-chloride cotransport". Physiological Reviews. 80 (1): 211–276. doi:10.1152/physrev.2000.80.1.211. ISSN 0031-9333. PMID 10617769. S2CID 8909659.
- ↑ Haas M, Forbush B (2000). "The Na-K-Cl cotransporter of secretory epithelia". Annu. Rev. Physiol. 62: 515–34. doi:10.1146/annurev.physiol.62.1.515. PMID 10845101.
- 1 2 Delpire E, Lu J, England R, Dull C, Thorne T (June 1999). "Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter". Nat. Genet. 22 (2): 192–5. doi:10.1038/9713. PMID 10369265. S2CID 23779936.
- ↑ Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ (November 2005). "NKCC1 transporter facilitates seizures in the developing brain". Nat. Med. 11 (11): 1205–13. doi:10.1038/nm1301. PMID 16227993. S2CID 25348736.
- ↑ Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R (October 2007). "GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations". Physiol. Rev. 87 (4): 1215–84. doi:10.1152/physrev.00017.2006. PMID 17928584.
- ↑ Lytle C, Xu JC, Biemesderfer D, Forbush B (December 1995). "Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies". Am. J. Physiol. 269 (6 Pt 1): C1496–505. doi:10.1152/ajpcell.1995.269.6.C1496. PMID 8572179.
- ↑ Gamba G, Friedman PA (May 2009). "Thick ascending limb: the Na(+):K (+):2Cl (-) co-transporter, NKCC2, and the calcium-sensing receptor, CaSR". Pflügers Arch. 458 (1): 61–76. doi:10.1007/s00424-008-0607-1. PMC 3584568 . PMID 18982348.
- ↑ Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V (January 2013). "Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC". Am. J. Pathol. 182 (1): 96–106. doi:10.1016/j.ajpath.2012.09.014. PMC 3532715 . PMID 23123217.
- ↑ Ares GR, Caceres PS, Ortiz PA (December 2011). "Molecular regulation of NKCC2 in the thick ascending limb". Am. J. Physiol. Renal Physiol. 301 (6): F1143–59. doi:10.1152/ajprenal.00396.2011. PMC 3233874 . PMID 21900458.
- ↑ Payne JA, Xu JC, Haas M, Lytle CY, Ward D, Forbush B (July 1995). "Primary structure, functional expression, and chromosomal localization of the bumetanide-sensitive Na-K-Cl cotransporter in human colon". J. Biol. Chem. 270 (30): 17977–85. doi: 10.1074/jbc.270.30.17977 . PMID 7629105.
- 1 2 Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP (June 1996). "Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2". Nat. Genet. 13 (2): 183–8. doi:10.1038/ng0696-183. PMID 8640224. S2CID 42296304.
- ↑ Plata C, Meade P, Vazquez N, Hebert SC, Gamba G (Mar 2002). "Functional properties of the apical Na+-K+-2Cl- cotransporter isoforms". J. Biol. Chem. 277 (13): 11004–12. doi: 10.1074/jbc.M110442200 . PMID 11790783.