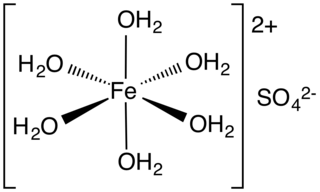
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for hydrated sulfate minerals), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

Lead(II) sulfate (PbSO4) is a white solid, which appears white in microcrystalline form. It is also known as fast white, milk white, sulfuric acid lead salt or anglesite.

Copper(II) sulfate is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, and Roman vitriol. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Tetraamminecopper(II) sulfate monohydrate, or more precisely tetraammineaquacopper(II) sulfate, is the salt with the formula [Cu(NH3)4]SO4·H2O, or more precisely [Cu(NH3)4(H2O)]SO4. This dark blue to purple solid is a sulfuric acid salt of the metal complex [Cu(NH3)4(H2O)]2+ (tetraammineaquacopper(II) cation). It is closely related to Schweizer's reagent, which is used for the production of cellulose fibers in the production of rayon.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".

Mercury(II) sulfate, commonly called mercuric sulfate, is the chemical compound HgSO4. It is an odorless salt that forms white granules or crystalline powder. In water, it separates into an insoluble basic sulfate with a yellow color and sulfuric acid.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.

Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble turquoise coloured salt is a common source of the Ni2+ ion for electroplating. Approximately 40,000 tonnes were produced in 2005.

Ammonium cerium(IV) sulfate is an inorganic compound with the formula (NH4)4Ce(SO4)4·2H2O. It is an orange-colored solid. It is a strong oxidant, the potential for reduction is about +1.44V. Cerium(IV) sulfate is a related compound.

Mercury(I) sulfate, commonly called mercurous sulphate (UK) or mercurous sulfate (US) is the chemical compound Hg2SO4. Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin.
Tutton's salts are a family of salts with the formula M2M'(SO4)2(H2O)6 (sulfates) or M2M'(SeO4)2(H2O)6 (selenates). These materials are double salts, which means that they contain two different cations, M+ and M'2+ crystallized in the same regular ionic lattice. The univalent cation can be potassium, rubidium, caesium, ammonium (NH4), deuterated ammonium (ND4) or thallium. Sodium or lithium ions are too small. The divalent cation can be magnesium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc or cadmium. In addition to sulfate and selenate, the divalent anion can be chromate (CrO42−), tetrafluoroberyllate (BeF42−), hydrogenphosphate (HPO42−) or monofluorophosphate (PO3F2−). Tutton's salts crystallize in the monoclinic space group P21/a. The robustness is the result of the complementary hydrogen-bonding between the tetrahedral anions and cations as well their interactions with the metal aquo complex [M(H2O)6]2+.

Cobalt(II) sulfate is any of the inorganic compounds with the formula CoSO4(H2O)x. Usually cobalt sulfate refers to the hexa- or heptahydrates CoSO4.6H2O or CoSO4.7H2O, respectively. The heptahydrate is a red solid that is soluble in water and methanol. Since cobalt(II) has an odd number of electrons, its salts are paramagnetic.
Langbeinites are a family of crystalline substances based on the structure of langbeinite with general formula M2M'2(SO4)3, where M is a large univalent cation, and M' is a small divalent cation. The sulfate group, SO2−4, can be substituted by other tetrahedral anions with a double negative charge such as tetrafluoroberyllate, selenate, chromate, molybdate, or tungstates. Although monofluorophosphates are predicted, they have not been described. By redistributing charges other anions with the same shape such as phosphate also form langbeinite structures. In these the M' atom must have a greater charge to balance the extra three negative charges.

Monofluorophosphate is an anion with the formula PO3F2−, which is a phosphate group with one oxygen atom substituted with a fluoride atom. The charge of the ion is −2. The ion resembles sulfate in size, shape and charge, and can thus form compounds with the same structure as sulfates. These include Tutton's salts and langbeinites. The most well-known compound of monofluorophosphate is sodium monofluorophosphate, commonly used in toothpaste.

Vanadium(II) sulfate describes a family of inorganic compounds with the formula VSO4(H2O)x where 0 ≤ x ≤ 7. The hexahydrate is most commonly encountered. It is a violet solid that dissolves in water to give air-sensitive solutions of the aquo complex. The salt is isomorphous with [Mg(H2O)6]SO4. Compared to the V–O bond length of 191 pm in [V(H2O)6]3+, the V–O distance is 212 pm in the [V(H2O)6]SO4. This nearly 10% elongation reflects the effect of the lower charge, hence weakened electrostatic attraction.
Copper(II) chlorate is a chemical compound of the transition metal copper and the chlorate anion with basic formula Cu(ClO3)2. Copper chlorate is an oxidiser. It commonly forms the tetrahydrate, Cu(ClO3)2·4H2O.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.
















