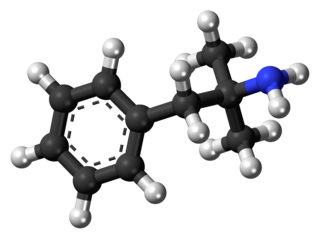
Phentermine, sold under the brand name Adipex-P among others, is a medication used together with diet and exercise to treat obesity. It is available by itself or as the combination phentermine/topiramate. Phentermine is taken by mouth.
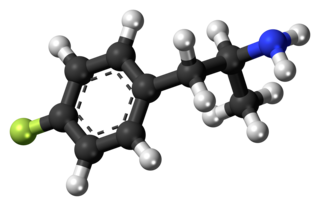
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

Chlorphentermine, sold under the brand names Apsedon, Desopimon, and Lucofen, is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the para-chloro derivative of the better-known appetite suppressant phentermine, which is still in current use.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.

MDAI, also known as 5,6-methylenedioxy-2-aminoindane, is an entactogen drug of the 2-aminoindane group which is related to MDMA and produces similar subjective effects.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters.
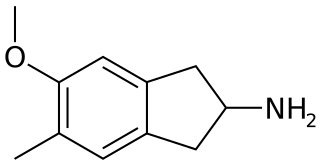
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug of the 2-aminoindane group developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a less neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogenic effects in humans. It has been sold as a designer drug and research chemical online since 2010.

A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons, including dopamine and norepinephrine neurons.

A serotonin–dopamine releasing agent (SDRA) is a type of drug which induces the release of serotonin and dopamine in the body and/or brain.

4-Methylmethamphetamine (4-MMA), also known as mephedrine, is a putative stimulant and entactogen drug of the amphetamine family. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). The drug is the β-deketo analogue of mephedrone and the N-methyl analogue of 4-methylamphetamine (4-MA).

para-Bromoamphetamine (PBA), also known as 4-bromoamphetamine (4-BA), is an amphetamine derivative which acts as a serotonin-norepinephrine-dopamine releasing agent (SNDRA) and produces stimulant effects.

para-Iodoamphetamine (PIA), also known as 4-iodoamphetamine (4-IA), is a monoamine releasing agent (MRA) and serotonergic neurotoxin of the amphetamine family related to para-chloroamphetamine (PCA).

o-Phenyl-3-iodotyramine (o-PIT) is a drug which acts as a selective agonist for the trace amine-associated receptor 1 (TAAR1). It has reasonable selectivity for TAAR1 but relatively low potency, and is rapidly metabolised in vivo, making it less useful for research than newer ligands such as RO5166017. Its EC50Tooltip half-maximal effective concentration values have been reported to be 35 nM for the mouse TAAR1, 2.4 nM at the rat TAAR1, and 9.5 nM at the human TAAR1.

5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative related to α-methyltryptamine (αMT) and one of only a few known serotonin–dopamine releasing agents (SDRAs). It is also a potent serotonin 5-HT2A receptor agonist and hence may be a serotonergic psychedelic. The drug has been investigated in animals as a potential treatment for cocaine dependence.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.
Monoaminergic activity enhancers (MAE), also known as catecholaminergic/serotonergic activity enhancers (CAE/SAE), are a class of drugs that enhance the action potential-evoked release of monoamine neurotransmitters in the nervous system. MAEs are distinct from monoamine releasing agents (MRAs) like amphetamine and fenfluramine in that they do not induce the release of monoamines from synaptic vesicles but rather potentiate only nerve impulse propagation-mediated monoamine release. That is, MAEs increase the amounts of monoamine neurotransmitters released by neurons per electrical impulse.

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine.

3-Chloroamphetamine, also known as meta-chloroamphetamine (MCA), is a psychostimulant of the amphetamine family and a serotonergic neurotoxin related to para-chloroamphetamine.

para-Bromomethamphetamine, also known as 4-bromomethamphetamine (4-BMA), is a monoaminergic drug of the amphetamine family related to para-chloroamphetamine. It was studied by József Knoll and colleagues in the 1970s and 1980s.

N,N-Dimethyl-4-methylthioamphetamine, also known as 4-methylthio-N,N-dimethylamphetamine (4-MTDMA), is a monoamine releasing agent (MRA) of the amphetamine family related to 4-methylthioamphetamine (4-MTA) and 4-methylthiomethamphetamine.




















