
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which shares many physical and chemical properties with the other five alkaline earth metals.
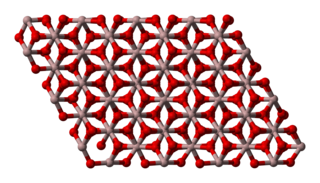
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium(III) oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum depending on particular forms or applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Sintering or frittage is the process of compacting and forming a solid mass of material by heat or pressure without melting it to the point of liquefaction.
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) in molten cryolite, and electrolyzing the molten salt bath, typically in a purpose-built cell. The Hall–Héroult process applied at industrial scale happens at 940–980 °C and produces 99.5–99.8% pure aluminium. Recycled aluminum requires no electrolysis, thus it does not end up in this process. This process contributes to climate change through the emission of carbon dioxide and fluorocarbons in the electrolytic reaction and consumption of large amounts of electrical energy.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be purified before it can be refined to aluminium metal.

Brazing is a metal-joining process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal.

A refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, inorganic, non-metallic, porous, and heterogeneous. They are typically composed of oxides or carbides, nitrides etc. of the following materials: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminum atom.

A foundry is a factory that produces metal castings. Metals are cast into shapes by melting them into a liquid, pouring the metal into a mold, and removing the mold material after the metal has solidified as it cools. The most common metals processed are aluminum and cast iron. However, other metals, such as bronze, brass, steel, magnesium, and zinc, are also used to produce castings in foundries. In this process, parts of desired shapes and sizes can be formed.

Plasma electrolytic oxidation (PEO), also known as electrolytic plasma oxidation (EPO) or microarc oxidation (MAO), is an electrochemical surface treatment process for generating oxide coatings on metals. It is similar to anodizing, but it employs higher potentials, so that discharges occur and the resulting plasma modifies the structure of the oxide layer. This process can be used to grow thick, largely crystalline, oxide coatings on metals such as aluminium, magnesium and titanium. Because they can present high hardness and a continuous barrier, these coatings can offer protection against wear, corrosion or heat as well as electrical insulation.
Spray forming, also known as spray casting, spray deposition and in-situ compaction, is a method of casting near net shape metal components with homogeneous microstructures via the deposition of semi-solid sprayed droplets onto a shaped substrate. In spray forming an alloy is melted, normally in an induction furnace, then the molten metal is slowly poured through a conical tundish into a small-bore ceramic nozzle. The molten metal exits the furnace as a thin free-falling stream and is broken up into droplets by an annular array of gas jets, and these droplets then proceed downwards, accelerated by the gas jets to impact onto a substrate. The process is arranged such that the droplets strike the substrate whilst in the semi-solid condition, this provides sufficient liquid fraction to 'stick' the solid fraction together. Deposition continues, gradually building up a spray formed billet of metal on the substrate.
Non-metallic inclusions are chemical compounds and nonmetals that are present in steel and other alloys. They are the product of chemical reactions, physical effects, and contamination that occurs during the melting and pouring process. These inclusions are categorized by origin as either endogenous or exogenous. Endogenous inclusions, also known as indigenous, occur within the metal and are the result of chemical reactions. These products precipitate during cooling and are typically very small. Exogenous inclusions are caused by the entrapment of nonmetals. Their size varies greatly and their source can include slag, dross, flux residues, and pieces of the mold.
Ceramic foam is a tough foam made from ceramics. Manufacturing techniques include impregnating open-cell polymer foams internally with ceramic slurry and then firing in a kiln, leaving only ceramic material. The foams may consist of several ceramic materials such as aluminium oxide, a common high-temperature ceramic, and gets insulating properties from the many tiny air-filled voids within the material.

Aluminium smelting is the process of extracting aluminium from its oxide, alumina, generally by the Hall-Héroult process. Alumina is extracted from the ore bauxite by means of the Bayer process at an alumina refinery.
Semi-solid metal casting (SSM) is a near net shape variant of die casting. The process is used today with non-ferrous metals, such as aluminium, copper, and magnesium, but also can work with higher temperature alloys for which no currently suitable die materials are available. The process combines the advantages of casting and forging. The process is named after the fluid property thixotropy, which is the phenomenon that allows this process to work. Simply, thixotropic fluids flow when sheared, but thicken when standing. The potential for this type of process was first recognized in the early 1970s. There are three different processes: thixocasting, rheocasting, thixomolding. SIMA refers to a specialized process to prepare aluminum alloys for thixocasting using hot and cold working.
A casting defect is an undesired irregularity in a metal casting process. Some defects can be tolerated while others can be repaired, otherwise they must be eliminated. They are broken down into five main categories: gas porosity, shrinkage defects, mould material defects, pouring metal defects, and metallurgical defects.
Hydrogen gas porosity is an aluminium casting defect in the form of a porosity or void in an aluminium casting caused by a high level of hydrogen gas (H2) dissolved in the aluminium at liquid phase. The solubility of hydrogen in solid aluminium is much smaller than in liquid aluminium. As the aluminium freezes, some of the hydrogen comes out of solution and forms bubbles, creating porosity in the solid aluminium.

Nanosized aluminium oxide occurs in the form of spherical or nearly spherical nanoparticles, and in the form of oriented or undirected fibers.

Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.












