The Alismatales (alismatids) are an order of flowering plants including about 4,500 species. Plants assigned to this order are mostly tropical or aquatic. Some grow in fresh water, some in marine habitats. Perhaps the most important food crop in the order is the corm of the taro plant, Colocasia esculenta.

Hydrocharitaceae is a flowering plant family including 16 known genera with a total of ca 135 known species, that including a number of species of aquatic plant, for instance the tape-grasses, the well known Canadian waterweed, and frogbit.

The Potamogetonaceae, commonly referred to as the pondweed family, is an aquatic family of monocotyledonous flowering plants. The roughly 110 known species are divided over six genera. The largest genus in the family by far is Potamogeton, which contains about 100 species.

Zosteraceae is a family of marine perennial flowering plants found in temperate and subtropical coastal waters, with the highest diversity located around Korea and Japan. Most seagrasses complete their entire life cycle under water, having filamentous pollen especially adapted to dispersion in an aquatic environment and ribbon-like leaves that lack stomata. Seagrasses are herbaceous and have prominent creeping rhizomes. A distinctive characteristic of the family is the presence of characteristic retinacules, which are present in all species except members of Zostera subgenus Zostera.
Antitropicaldistribution is a type of disjunct distribution where a species or clade exists at comparable latitudes across the equator but not in the tropics. For example, a species may be found north of the Tropic of Cancer and south of the Tropic of Capricorn, but not in between. With increasing time since dispersal, the disjunct populations may be the same variety, species, or clade. How the life forms distribute themselves to the opposite hemisphere when they can't normally survive in the middle depends on the species; plants may have their seed spread through wind, animal, or other methods and then germinate upon reaching the appropriate climate, while sea life may be able to travel through the tropical regions in a larval state or by going through deep ocean currents with much colder temperatures than on the surface. For the American amphitropical distribution, dispersal has been generally agreed to be more likely than vicariance from a previous distribution including the tropics in North and South America.

Chrysobalanaceae is a family of flowering plants, consisting of trees and shrubs in 27 genera and about 700 species of pantropical distribution with a centre of diversity in the Amazon. Some of the species contain silica in their bodies for rigidity and so the mesophyll often has sclerenchymatous idioblasts. The widespread species Chrysobalanus icaco produces a plum-like fruit and the plant is commonly known as the coco plum.

Najadales is the botanical name of an order of flowering plants. A well-known system that used this name is the Cronquist system (1981), which used this name for an order in subclass Alismatidae with this circumscription:

Cymodoceaceae is a family of flowering plants, sometimes known as the "manatee-grass family", which includes only marine species.

The Anarthriaceae was a family of three genera, Anarthria, Hopkinsia and Lyginia of flowering plants, now included in Restionaceae following APG IV (2016). The family is accepted in the Angiosperm Phylogeny Group's classification system, APG III system, but is not considered a separate family in many other taxonomic systems. The three genera are herbaceous but differ greatly in characteristics.

Nobuyuki Tanaka is an economic botanist at the Tokyo Metropolitan University, the Makino Botanical Garden in Kōchi Prefecture, Japan.

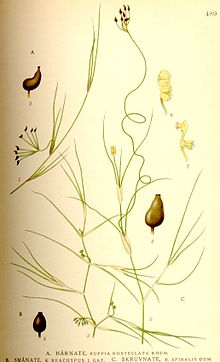
Ruppia cirrhosa is a species of aquatic plant known by the common names spiral ditchgrass and spiral tasselweed. It is native to the Americas and Europe, where it grows in freshwater bodies, such as lakes. It is a thread-thin, grasslike perennial herb which grows from a rhizome anchored in the wet substrate. It produces a long, narrow inflorescence tipped with two tiny flowers. As the fruit develops the peduncle of the inflorescence curls into a neat spiral.

Ruppia maritima is an aquatic plant species commonly known as beaked tasselweed, beaked ditchgrass, ditch grass, tassel pondweed and widgeon grass. Despite its scientific name, it is not a marine plant; is perhaps best described as a salt-tolerant freshwater species. The generic name Ruppia was dedicated by Linnaeus to the German botanist Heinrich Bernhard Ruppius (1689–1719) and the specific name (maritima) translates to "of the sea".
Ruppia megacarpa is a submerged herb species in the genus Ruppia found in shallow brackish waters. It is a common on Australasian coasts, including Australia (NSW; SA; Vic; WA and New Zealand. Isolated populations have been currently found in East Asia, including Japan, Korea, and Far East Russia, hence, the species distribution exhibit latitudinally disjunct distribution between East Asia and Australasia.

Ruppia tuberosa is a submerged herb in the genus Ruppia found in shallow hypersaline waters in Australia.
Chloroplast capture is an evolutionary process through which inter-species hybridization and subsequent backcrosses yield a plant with new genetic combination of nuclear and chloroplast genomes. For instance, 1) species A's pollen hybridizes (backcross) to species B's ovule, yielding the 1st hybrid (F1) with chloroplast genome b and nuclear genome A (50%) and B (50%); 2) species A's pollen again hybridizes (backcross) to F1's ovule, yielding the 2nd hybrid (F2) with chloroplast genome b and nuclear genome A (75%) and B (25%); 3) species A's pollen again hybridizes (backcross) to F2's ovule, yielding the 3rd hybrid (F3) with chloroplast genome b and nuclear genome A (87.5%) and B (12.5%); 4) after further backcross generations, a plant is obtained with the new genetic combination.
Norio Tanaka is an aquatic botanist at Tsukuba Botanical Garden, National Science Museum, Tokyo, Japan.

Najas tenuis is a species of aquatic plant found in freshwater habitats, especially still or slow-moving waters, like ponds and rice fields.
Ruppia bicarpa is an aquatic plant species in the genus Ruppia of Ruppiaceae. It is found in shallow waters.
Ruppia spiralis is an aquatic plant species in the genus Ruppia of the family Ruppiaceae. This name was synonymized under R. cirrhosa, but it has been resurrected for the species previously referred to as R. cirrhosa.