
Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands:

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.
A drug with psychotomimetic actions mimics the symptoms of psychosis, including delusions and/or delirium, as opposed to only hallucinations. Psychotomimesis is the onset of psychotic symptoms following the administration of such a drug.
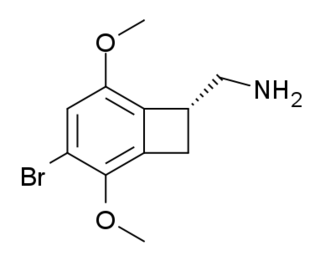
TCB-2 is a hallucinogen discovered in 2006 by Thomas McLean working in the lab of David Nichols at Purdue University. It is a conformationally-restricted derivative of the phenethylamine 2C-B, also a hallucinogen, and acts as a potent agonist for the 5-HT2A and 5-HT2C receptors with a Ki of 0.26 nM at the human 5-HT2A receptor.

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

Metapramine is a tricyclic antidepressant (TCA) developed by Rhone Poulenc that was introduced for the treatment of depression in France in 1984. In addition to its efficacy against affective disorders, it also has analgesic properties, and may be useful in the treatment of pain.

HU-320 (7-nor-7-carboxy-CBD-1,1-DMH) is a drug related to cannabidiol, which has strong antiinflammatory and immunosuppressive properties while demonstrating no psychoactive effects.

Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative.
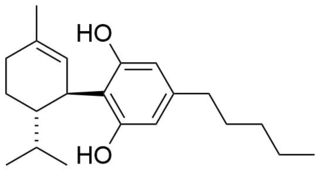
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

Cannabidiphorol, the heptyl-homologue of cannabidiol was identified as a natural phytocannabinoid and named cannabidiphorol (CBDP) in 2019. It had previously been reported as a synthetic compound, but was not identified as a natural product prior to 2019. Recently, CBDP has been gained popularity due to it being synthesized and available on a commercial level.

7-Hydroxycannabidiol (7-OH-CBD) is an active metabolite of cannabidiol, generated in the body from cannabidiol by the action of the enzyme CYP2C19. While methods have been developed for its synthetic production, and measurement of levels in the body following consumption of cannabidiol, its pharmacology has been relatively little studied, though it has been found to possess similar anticonvulsant effects to cannabidiol itself, as well as lowering blood triglyceride levels. Like its precursor CBD, it is not known to exhibit any psychoactive effects on the body and is known to counter the psychoactive effects of THC if it is present at the same time. This mode of action in 2015 was discovered to be at least contributing in part by being a non competitive negative allosteric modulator of the Cannabinoid receptor type 1.

Cannabidiol dimethyl ether (CBDD) is a trace component of cannabis which can also be made synthetically. It is a potent and selective inhibitor of the enzyme 15-lipoxygenase and inhibits oxygenation of linoleic acid, a process involved in the development of atherosclerosis.

H4CBD is a cannabinoid that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

H2CBD are cannabinoids that were first synthesized by Alexander R. Todd in 1940 by catalytic hydrogenation of cannabidiol.

Etrinabdione (VCE-004.8, EHP-101) is a drug structurally related to cannabidiol and HU-331 which has potent antiinflammatory and neuroprotective effects. It acts as a dual agonist of CB2 and PPARγ and is in clinical trials for the treatment of scleroderma.
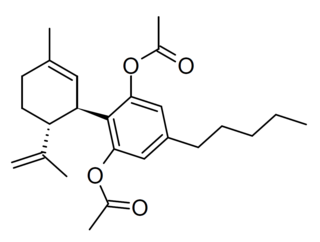
Cannabidiol diacetate is a semi-synthetic derivative of cannabidiol derived by acetylation of the OH groups, which presumably acts as a prodrug for CBD. It has been found as a component of grey-market cannabis products such as e-cigarette liquids and edible gummy lollies.


















