Related Research Articles

A primer is a short single-stranded nucleic acid used by all living organisms in the initiation of DNA synthesis. DNA polymerase enzymes are only capable of adding nucleotides to the 3’-end of an existing nucleic acid, requiring a primer be bound to the template before DNA polymerase can begin a complementary strand. DNA polymerase adds nucleotides after binding to the RNA primer and synthesizes the whole strand. Later, the RNA strands must be removed accurately and replace them with DNA nucleotides forming a gap region known as a nick that is filled in using an enzyme called ligase. The removal process of the RNA primer requires several enzymes, such as Fen1, Lig1, and others that work in coordination with DNA polymerase, to ensure the removal of the RNA nucleotides and the addition of DNA nucleotides. Living organisms use solely RNA primers, while laboratory techniques in biochemistry and molecular biology that require in vitro DNA synthesis usually use DNA primers, since they are more temperature stable. Primers can be designed in laboratory for specific reactions such as polymerase chain reaction (PCR). When designing PCR primers, there are specific measures that must be taken into consideration, like the melting temperature of the primers and the annealing temperature of the reaction itself. Moreover, the DNA binding sequence of the primer in vitro has to be specifically chosen, which is done using a method called basic local alignment search tool (BLAST) that scans the DNA and finds specific and unique regions for the primer to bind.
A restriction enzyme, restriction endonuclease, REase, ENase orrestrictase is an enzyme that cleaves DNA into fragments at or near specific recognition sites within molecules known as restriction sites. Restriction enzymes are one class of the broader endonuclease group of enzymes. Restriction enzymes are commonly classified into five types, which differ in their structure and whether they cut their DNA substrate at their recognition site, or if the recognition and cleavage sites are separate from one another. To cut DNA, all restriction enzymes make two incisions, once through each sugar-phosphate backbone of the DNA double helix.

A nuclease is an enzyme capable of cleaving the phosphodiester bonds between nucleotides of nucleic acids. Nucleases variously effect single and double stranded breaks in their target molecules. In living organisms, they are essential machinery for many aspects of DNA repair. Defects in certain nucleases can cause genetic instability or immunodeficiency. Nucleases are also extensively used in molecular cloning.

Okazaki fragments are short sequences of DNA nucleotides which are synthesized discontinuously and later linked together by the enzyme DNA ligase to create the lagging strand during DNA replication. They were discovered in the 1960s by the Japanese molecular biologists Reiji and Tsuneko Okazaki, along with the help of some of their colleagues.

Exodeoxyribonuclease V is an enzyme of E. coli that initiates recombinational repair from potentially lethal double strand breaks in DNA which may result from ionizing radiation, replication errors, endonucleases, oxidative damage, and a host of other factors. The RecBCD enzyme is both a helicase that unwinds, or separates the strands of DNA, and a nuclease that makes single-stranded nicks in DNA. It catalyses exonucleolytic cleavage in either 5′- to 3′- or 3′- to 5′-direction to yield 5′-phosphooligonucleotides.
Endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain. Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically, while many, typically called restriction endonucleases or restriction enzymes, cleave only at very specific nucleotide sequences. Endonucleases differ from exonucleases, which cleave the ends of recognition sequences instead of the middle (endo) portion. Some enzymes known as "exo-endonucleases", however, are not limited to either nuclease function, displaying qualities that are both endo- and exo-like. Evidence suggests that endonuclease activity experiences a lag compared to exonuclease activity.

Exonucleases are enzymes that work by cleaving nucleotides one at a time from the end (exo) of a polynucleotide chain. A hydrolyzing reaction that breaks phosphodiester bonds at either the 3′ or the 5′ end occurs. Its close relative is the endonuclease, which cleaves phosphodiester bonds in the middle (endo) of a polynucleotide chain. Eukaryotes and prokaryotes have three types of exonucleases involved in the normal turnover of mRNA: 5′ to 3′ exonuclease (Xrn1), which is a dependent decapping protein; 3′ to 5′ exonuclease, an independent protein; and poly(A)-specific 3′ to 5′ exonuclease.
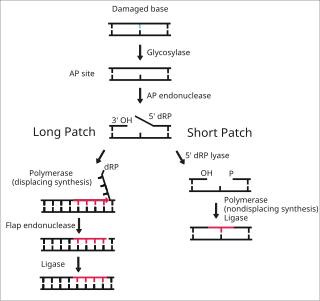
Base excision repair (BER) is a cellular mechanism, studied in the fields of biochemistry and genetics, that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from the genome. The related nucleotide excision repair pathway repairs bulky helix-distorting lesions. BER is important for removing damaged bases that could otherwise cause mutations by mispairing or lead to breaks in DNA during replication. BER is initiated by DNA glycosylases, which recognize and remove specific damaged or inappropriate bases, forming AP sites. These are then cleaved by an AP endonuclease. The resulting single-strand break can then be processed by either short-patch or long-patch BER.
Micrococcal nuclease is an endo-exonuclease that preferentially digests single-stranded nucleic acids. The rate of cleavage is 30 times greater at the 5' side of A or T than at G or C and results in the production of mononucleotides and oligonucleotides with terminal 3'-phosphates. The enzyme is also active against double-stranded DNA and RNA and all sequences will be ultimately cleaved.
A nick is a discontinuity in a double stranded DNA molecule where there is no phosphodiester bond between adjacent nucleotides of one strand typically through damage or enzyme action. Nicks allow DNA strands to untwist during replication, and are also thought to play a role in the DNA mismatch repair mechanisms that fix errors on both the leading and lagging daughter strands.
Exonuclease III (ExoIII) is an enzyme that belongs to the exonuclease family. ExoIII catalyzes the stepwise removal of mononucleotides from 3´-hydroxyl termini of double-stranded DNA. A limited number of nucleotides are removed during each binding event, resulting in coordinated progressive deletions within the population of DNA molecules.
Mung bean nuclease is a nuclease derived from sprouts of the mung bean that removes nucleotides in a step-wise manner from single-stranded DNA molecules (ssDNA) and is used in biotechnological applications to remove such ssDNA from a mixture also containing double-stranded DNA (dsDNA). This enzyme is useful for transcript mapping, removal of single-stranded regions in DNA hybrids or single-stranded overhangs produced by restriction enzymes, etc. It has an activity similar to Nuclease S1, but it has higher specificity for single-stranded molecules.

The restriction endonuclease Fok1, naturally found in Flavobacterium okeanokoites, is a bacterial type IIS restriction endonuclease consisting of an N-terminal DNA-binding domain and a non-specific DNA cleavage domain at the C-terminal. Once the protein is bound to duplex DNA via its DNA-binding domain at the 5'-GGATG-3' recognition site, the DNA cleavage domain is activated and cleaves, without further sequence specificity, the first strand 9 nucleotides downstream and the second strand 13 nucleotides upstream of the nearest nucleotide of the recognition site.
SNP genotyping is the measurement of genetic variations of single nucleotide polymorphisms (SNPs) between members of a species. It is a form of genotyping, which is the measurement of more general genetic variation. SNPs are one of the most common types of genetic variation. An SNP is a single base pair mutation at a specific locus, usually consisting of two alleles. SNPs are found to be involved in the etiology of many human diseases and are becoming of particular interest in pharmacogenetics. Because SNPs are conserved during evolution, they have been proposed as markers for use in quantitative trait loci (QTL) analysis and in association studies in place of microsatellites. The use of SNPs is being extended in the HapMap project, which aims to provide the minimal set of SNPs needed to genotype the human genome. SNPs can also provide a genetic fingerprint for use in identity testing. The increase of interest in SNPs has been reflected by the furious development of a diverse range of SNP genotyping methods.

Nuclease S1 is an endonuclease enzyme that splits single-stranded DNA (ssDNA) and RNA into oligo- or mononucleotides. This enzyme catalyses the following chemical reaction
Deoxyribonuclease IV (phage-T4-induced) is a kind of Endonuclease that catalyzes the degradation nucleotides in DsDNA by attacking the 5'-terminal end.

Flap endonuclease 1 is an enzyme that in humans is encoded by the FEN1 gene.

Cas9 is a 160 kilodalton protein which plays a vital role in the immunological defense of certain bacteria against DNA viruses and plasmids, and is heavily utilized in genetic engineering applications. Its main function is to cut DNA and thereby alter a cell's genome. The CRISPR-Cas9 genome editing technique was a significant contributor to the Nobel Prize in Chemistry in 2020 being awarded to Emmanuelle Charpentier and Jennifer Doudna.

Surveyor nuclease assay is an enzyme mismatch cleavage assay used to detect single base mismatches or small insertions or deletions (indels).

Mitochondrial genome maintenance exonuclease 1, abbreviated as MGME1, is an enzyme that in humans is encoded by the MGME1 gene. MGME1 is a 344 amino acids long protein belonging to the PD-(D/E)XK family of nucleases. It localizes to mitochondria where it is important for maintenance of the mitochondrial genome. Loss of function mutations in MGME1 lead to defects in mitochondrial DNA, including mitochondrial DNA depletion, duplications, deletions and increased replication intermediates. Also, there is an accumulation of 7S DNA, a short single stranded linear DNA strand. MGME1 deficiency in humans leads to multisystemic mitochondrial disease.
References
- ↑ Sayers, Jon R. (1994). "Computer Aided Identification of a Potential 5′-3′ Exonuclease Gene Encoded by Escherichia coli". Journal of Theoretical Biology. 170 (4): 415–21. doi:10.1006/jtbi.1994.1202. PMID 7996866.
- ↑ Liu, Yuan; Kao, Hui-I; Bambara, Robert A. (2004). "FLAP ENDONUCLEASE 1: A Central Component of DNA Metabolism". Annual Review of Biochemistry. 73: 589–615. doi:10.1146/annurev.biochem.73.012803.092453. PMID 15189154.
- ↑ Ceska, T; Sayers, JR (1998). "Structure-specific DNA cleavage by 5′ nucleases". Trends in Biochemical Sciences. 23 (9): 331–6. doi:10.1016/S0968-0004(98)01259-6. PMID 9787638.
- ↑ Lyamichev, Victor; Brow, Mary Ann D.; Dahlberg, James E. (1993). "Structure-specific endonucleolytic cleavage of nucleic acids by eubacterial DNA polymerases". Science. 260 (5109): 778–783. Bibcode:1993Sci...260..778L. doi:10.1126/science.7683443. PMID 7683443.
- ↑ Harrington, John J.; Lieber, Michael R. (1994). "The characterization of a mammalian DNA structure-specific endonuclease". The EMBO Journal. 13 (5): 1235–46. doi:10.1002/j.1460-2075.1994.tb06373.x. PMC 394933 . PMID 8131753.
- ↑ Kaiser, Michael W.; Lyamicheva, N.; Ma, W.; Miller, C.; Neri, B.; Fors, L.; Lyamichev, V. (1999). "A Comparison of Eubacterial and Archaeal Structure-specific 5′-Exonucleases". Journal of Biological Chemistry. 274 (30): 21387–21394. doi: 10.1074/jbc.274.30.21387 . PMID 10409700.
- ↑ Ceska, T. A.; Sayers, J. R.; Stier, G.; Suck, D. (1996). "A helical arch allowing single-stranded DNA to thread through T5 5'-exonuclease". Nature. 382 (6586): 90–3. Bibcode:1996Natur.382...90C. doi:10.1038/382090a0. PMID 8657312. S2CID 11159640.
- ↑ http://www.med.unc.edu/anclinic/Tm.htm%5B%5D
- ↑ Lyamichev, V.; Mast, A.L.; Hall, J.G.; Prudent, J.R.; Kaiser, M.W.; Takova, T.; Kwiatkowski, R.; Sander, T.; deArruda, M.; Arco, D.; Neri, B.P.; Brow, M.A. (1999). "Polymorphism identification and quantitative detection of genomic DNA by invasive cleavage of oligonucleotide probes". Nature Biotechnology. 17 (3): 292–296. doi:10.1038/7044. PMID 10096299. S2CID 37888925.
- ↑ Olivier, Michael (2005). "The Invader® assay for SNP genotyping". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 573 (1–2): 103–10. doi:10.1016/j.mrfmmm.2004.08.016. PMC 2771639 . PMID 15829241.