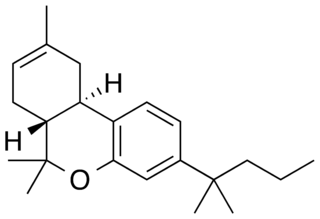
JWH-133(Dimethylbutyl-deoxy-Delta-8-THC) is a potent selective CB2 receptor agonist with a Ki of 3.4nM and selectivity of around 200x for CB2 over CB1 receptors. It was discovered by and named after, John W. Huffman.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).
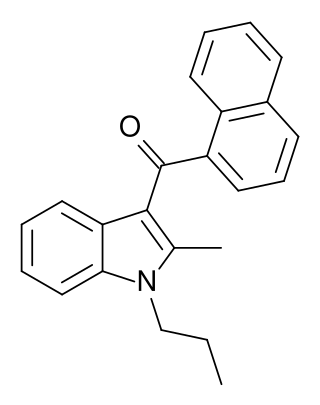
JWH-015 is a chemical from the naphthoylindole family that acts as a subtype-selective cannabinoid agonist. Its affinity for CB2 receptors is 13.8 nM, while its affinity for CB1 is 383 nM, meaning that it binds almost 28 times more strongly to CB2 than to CB1. However, it still displays some CB1 activity, and in some model systems can be very potent and efficacious at activating CB1 receptors, and therefore it is not as selective as newer drugs such as JWH-133. It has been shown to possess immunomodulatory effects, and CB2 agonists may be useful in the treatment of pain and inflammation. It was discovered and named after John W. Huffman.

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.
L-759,633 is an analgesic drug that is a cannabinoid agonist. It is a fairly selective agonist for the CB2 receptor, with selectivity of 163x for CB2 over CB1.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

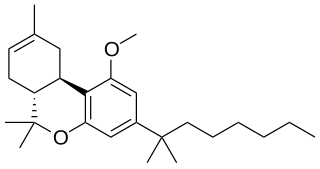
JWH-359 is a dibenzopyran "classical" cannabinoid drug, which is a potent and selective CB2 receptor agonist, with a Ki of 13.0 nM and selectivity of around 220 times for CB2 over CB1 receptors. It is related to other dibenzopyran CB2 agonists such as JWH-133 and L-759,656 but with a chiral side chain which has made it useful for mapping the shape of the CB2 binding site. It was discovered by, and named after, John W. Huffman.

GW-405,833 (L-768,242) is a drug that acts as a potent and selective partial agonist for the cannabinoid receptor subtype CB2, with an EC50 of 0.65 nM and selectivity of around 1200x for CB2 over CB1 receptors. Animal studies have shown it to possess antiinflammatory and anti-hyperalgesic effects at low doses, followed by ataxia and analgesic effects when the dose is increased. Selective CB2 agonist drugs such as GW-405,833 are hoped to be particularly useful in the treatment of allodynia and neuropathic pain for which current treatment options are often inadequate.

AM-1241 (1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole) is a chemical from the aminoalkylindole family that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 3.4 nM at CB2 and 80 times selectivity over the related CB1 receptor. It has analgesic effects in animal studies, particularly against "atypical" pain such as hyperalgesia and allodynia. This is thought to be mediated through CB2-mediated peripheral release of endogenous opioid peptides, as well as direct activation of the TRPA1 channel. It has also shown efficacy in the treatment of amyotrophic lateral sclerosis in animal models.

JWH-007 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It was first reported in 1994 by a group including the noted cannabinoid chemist John W. Huffman. It was the most active of the first group of N-alkyl naphoylindoles discovered by the team led by John W Huffman, several years after the family was initially described with the discovery of the N-morpholinylethyl compounds pravadoline (WIN 48,098), JWH-200 (WIN 55,225) and WIN 55,212-2 by the Sterling Winthrop group. Several other N-alkyl substituents were found to be active by Huffman's team including the n-butyl, n-hexyl, 2-heptyl, and cyclohexylethyl groups, but it was subsequently determined that the 2-methyl group on the indole ring is not required for CB1 binding, and tends to increase affinity for CB2 instead. Consequently, the 2-desmethyl derivative of JWH-007, JWH-018, has slightly higher binding affinity for CB1, with an optimum binding of 9.00 nM at CB1 and 2.94 nM at CB2, and JWH-007 displayed optimum binding of 9.50 nM at CB1 and 2.94 nM at CB2.

JWH-019 is an analgesic chemical from the naphthoylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is the N-hexyl homolog of the more common synthetic cannabinoid compound JWH-018. Unlike the butyl homolog JWH-073, which is several times weaker than JWH-018, the hexyl homolog is only slightly less potent, although extending the chain one carbon longer to the heptyl homolog JWH-020 results in dramatic loss of activity. These results show that the optimum side chain length for CB1 binding in the naphthoylindole series is the five-carbon pentyl chain, shorter than in the classical cannabinoids where a seven-carbon heptyl chain produces the most potent compounds. This difference is thought to reflect a slightly different binding conformation adopted by the naphthoylindole compounds as compared to the classical cannabinoids, and may be useful in characterizing the active site of the CB1 and CB2 receptors.
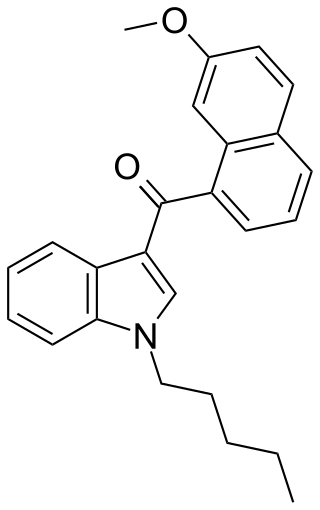
JWH-164 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It has approximately equal affinity for the CB1 and CB2 receptors, with a Ki of 6.6 nM at CB1 and 6.9 nM at CB2. JWH-164 is a positional isomer of the related compound JWH-081, but with a methoxy group at the 7-position of the naphthyl ring, rather than the 4-position as in JWH-081. Its potency is intermediate between that of JWH-081 and its ring unsubstituted derivative JWH-018, demonstrating that substitution of the naphthyl 7-position can also result in increased cannabinoid receptor binding affinity.

A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.
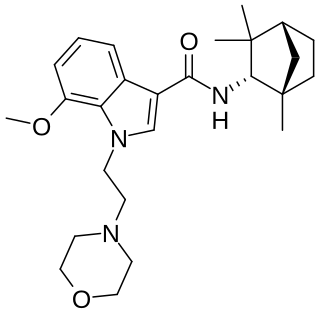
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).
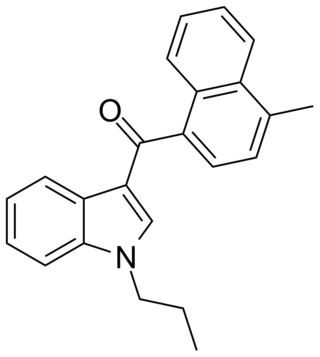
JWH-120 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-propyl analog of JWH-122. It is a potent and selective ligand for the CB2 receptor, but a weaker ligand for the CB1 receptor. It has a binding affinity of Ki = 6.1 ± 0.7 nM at the CB2 subtype and 173 times selectivity over the CB1 subtype.

JWH-148 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the indole 2-methyl analog of JWH-120. It is a moderately selective ligand for the CB2 receptor, with a binding affinity of Ki = 14.0 ± 1.0 nM at this subtype, and more than eight times selectivity over the CB1 subtype.
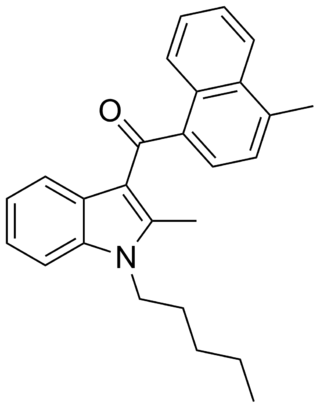
JWH-149 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-pentyl analog of JWH-148. It is a potent but only moderately selective ligand for the CB2 receptor, with a binding affinity of Ki = 0.73 ± 0.03 nM at this subtype, and more than six times selectivity over the CB1 subtype.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.
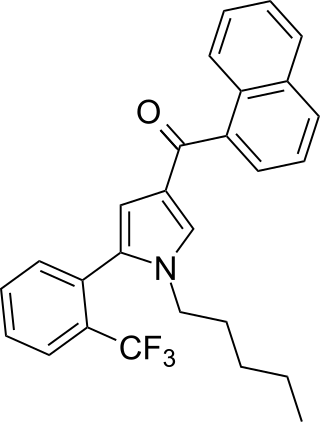
JWH-372 (naphthalen-1-yl-[1-pentyl-5-[2-(trifluoromethyl)phenyl]pyrrol-3-yl]methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent and selective agonist of the CB2 receptor. JWH-372 binds approximately 9 times stronger to the CB2 receptor (Ki = 8.2 ± 0.2nM) than the CB1 receptor (Ki = 77 ± 2nM). The selectivity of JWH-372 for the CB2 receptor is likely due to the electron-withdrawing character of the trifluoromethyl group rather than steric effects, as the o-methyl compound JWH-370 was only mildly selective for the CB2 receptor (CB1 Ki = 5.6 ± 0.4nM, CB2 Ki = 4.0 ± 0.5nM).


















