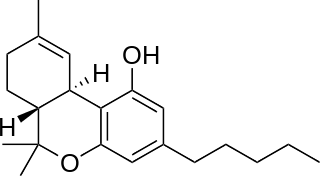
Tetrahydrocannabinol (THC) is a cannabinoid found in cannabis. It is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. It is a colorless oil.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.
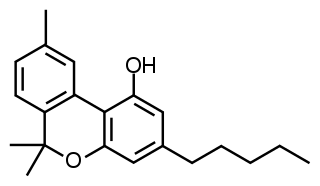
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

11-Hydroxy-Δ9-tetrahydrocannabinol, usually referred to as 11-hydroxy-THC is the main active metabolite of tetrahydrocannabinol (THC), which is formed in the body after Δ9-THC is consumed.

Dimethylheptylpyran (DMHP) is a synthetic cannabinoid and analogue of tetrahydrocannabinol (THC). It was invented in 1949 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. DMHP is a pale yellow, viscous oil which is insoluble in water but dissolves in alcohol or non-polar solvents.
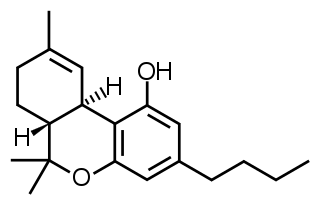
Δ9-Tetrahydrocannabutol is a phytocannabinoid found in cannabis that is a homologue of tetrahydrocannabinol (THC), the main active component of Cannabis. Structurally, they are only different by the pentyl side chain being replaced by a butyl side chain. THCB was studied by Roger Adams as early as 1942

11-Nor-9-carboxy-Δ9-tetrahydrocannabinol, often referred to as 11-nor-9-carboxy-THC or THC-11-oic acid, is the main secondary metabolite of tetrahydrocannabinol (THC) which is formed in the body after cannabis is consumed.

Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, exhibits anti-inflammatory properties in vitro, which may, theoretically, contribute to cannabis analgesic effects. It is a phytocannabinoid, one of the hundreds of cannabinoids found in the Cannabis plant. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and cannabinols are present in cannabis. It is not scheduled by the Convention on Psychotropic Substances.

Dronabinol, sold under the brand names Marinol and Syndros, is the generic name for the molecule of (−)-trans-Δ9-tetrahydrocannabinol (THC) in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the U.S. Food and Drug Administration (FDA) as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting.
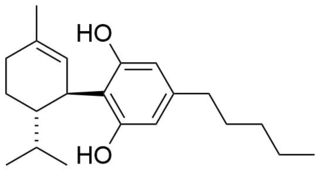
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

Δ-8-tetrahydrocannabinol is a psychoactive cannabinoid found in the cannabis plant. It is an isomer of delta-9-tetrahydrocannabinol, the compound commonly known as THC, with which it co-occurs in hemp; natural quantities of ∆8-THC found in hemp are low. Psychoactive effects are similar to that of Δ9-THC, with central effects occurring by binding to cannabinoid receptors found in various regions of the brain.

7-Hydroxycannabidiol (7-OH-CBD) is an active metabolite of cannabidiol, generated in the body from cannabidiol by the action of the enzyme CYP2C19. While methods have been developed for its synthetic production, and measurement of levels in the body following consumption of cannabidiol, its pharmacology has been relatively little studied, though it has been found to possess similar anticonvulsant effects to cannabidiol itself, as well as lowering blood triglyceride levels. Like its precursor CBD, it is not known to exhibit any psychoactive effects on the body and is known to counter the psychoactive effects of THC if it is present at the same time. This mode of action in 2015 was discovered to be at least contributing in part by being a non competitive negative allosteric modulator of the Cannabinoid receptor type 1.

Cannabielsoin (CBE) is a metabolite of cannabidiol, one of the major chemical components of cannabis.

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by firstly acid cyclization of cannabidiol and then hydrogenation of tetrahydrocannabinol. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams.

8,11-Dihydroxytetrahydrocannabinol (8β,11-diOH-Δ9-THC) is an active metabolite of THC, the main active component of cannabis. The 8β enantiomer retains psychoactive effects in animal studies with only slightly lower potency than THC, while the 8α enantiomer is much weaker. Both enantiomers have a shorter half-life in the body than 11-hydroxy-THC, making 8,11-dihydroxy-THC potentially useful for drug testing to distinguish between recent cannabis use and use longer in the past.

11-Hydroxy-Δ-8-tetrahydrocannabinol is an active metabolite of Δ8-THC, a psychoactive cannabinoid found in small amounts in cannabis. It is an isomer of 11-OH-Δ9-THC, and is produced via the same metabolic pathway. It was the first cannabinoid metabolite discovered in 1970.

3'-Hydroxy-THC (3'-OH-Δ9-THC) is a minor active metabolite of THC, the main psychoactive component of cannabis. It is one of a number of metabolites of THC hydroxylated on the pentyl side chain, but while the other side-chain hydroxyl isomers are much weaker or inactive, the S enantiomer of 3'-OH-THC is several times more potent than THC itself, and while it is produced in smaller amounts than other active metabolites such as 11-Hydroxy-THC and 8,11-Dihydroxy-THC, it is thought to contribute to the overall pharmacological profile of cannabis.

11-Hydroxyhexahydrocannabinol is an active metabolite of tetrahydrocannabinol (THC) and a metabolite of the trace cannabinoid hexahydrocannabinol (HHC).
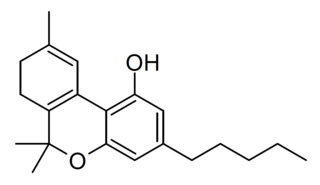
7,8-Dihydrocannabinol (7,8-DHC) is a trace component of cannabis. Despite its structural similarity to active cannabinoids such as tetrahydrocannabinol and cannabinol, its pharmacology has not been studied.
Cannabinoids are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is another major constituent of some cannabis plants. Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.


















