The compound 2-chlorobenzalmalononitrile (also called o-chlorobenzylidene malononitrile; chemical formula: C10H5ClN2), a cyanocarbon, is the defining component of the lachrymatory agent commonly referred to as CS gas, a tear gas which is used as a riot control agent, and is banned for use in warfare due to the 1925 Geneva Protocol.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.

Andre Kostelanetz was a Russian-American popular orchestral music conductor and arranger who was one of the major exponents of popular orchestra music.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
Chlorotoluenes are aryl chlorides based on toluene in which at least one aromatic hydrogen atom is replaced with a chlorine atom. They have the general formula C7H8–nCln, where n = 1–5 is the number of chlorine atoms.

Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.

Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.
In enzymology, a benzaldehyde dehydrogenase (NADP+) (EC 1.2.1.7) is an enzyme that catalyzes the chemical reaction
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.

Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:
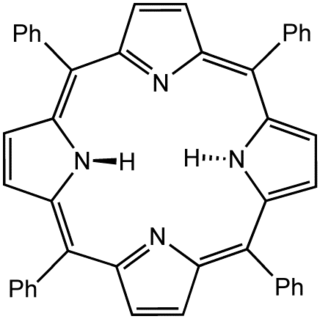
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.

3-Nitrobenzaldehyde is an organic compound with the formula O2NC6H4CHO. It is one of three isomers of nitrobenzaldehyde. It contains a nitro group meta-substituted to the aldehyde.

Buoyant density centrifugation uses the concept of buoyancy to separate molecules in solution by their differences in density.
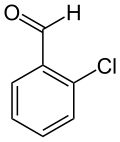
Chlorobenzaldehyde may refer to:
Fluorobenzaldehyde is a group of three constitutional isomers of fluorinated benzaldehyde.
4-Chlorobenzaldehyde (o-Chlorobenzaldehyde) is an organic compound with the formula ClC5H4CHO. It is one of three isomeric monochlorinated benzaldehydes.