
Orexin, also known as hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. The most common form of narcolepsy, in which the sufferer experiences brief losses of muscle tone (cataplexy), is caused by a lack of orexin in the brain due to destruction of the cells that produce it.

Doxepin is a medication used to treat major depressive disorder, anxiety disorders, chronic hives, and trouble sleeping. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.
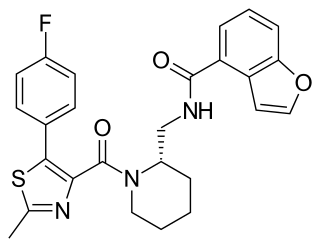
SB-649868 is a dual orexin receptor antagonist in development by GlaxoSmithKline. The drug is currently in phase II development for insomnia.
The orexin receptor (also referred to as the hypocretin receptor) is a G-protein-coupled receptor that binds the neuropeptide orexin. There are two variants, OX1 and OX2, each encoded by a different gene (HCRTR1, HCRTR2).

Orexin receptor type 1 (Ox1R or OX1), also known as hypocretin receptor type 1 (HcrtR1), is a protein that in humans is encoded by the HCRTR1 gene.

Orexin receptor type 2 (Ox2R or OX2), also known as hypocretin receptor type 2 (HcrtR2), is a protein that in humans is encoded by the HCRTR2 gene.

Narcolepsy is a long-term neurological disorder that involves a decreased ability to regulate sleep-wake cycles. Symptoms include periods of excessive daytime sleepiness that usually last from seconds to minutes and may occur at any time. About 70% of those affected also experience episodes of sudden loss of muscle strength, known as cataplexy. These experiences can be brought on by strong emotions. Less commonly, there may be inability to move or vivid hallucinations while falling asleep or waking up. People with narcolepsy tend to sleep about the same number of hours per day as people without, but the quality of sleep tends to be worse.
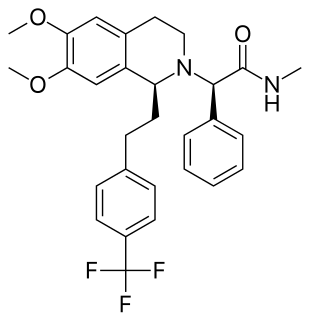
Almorexant (INN, codenamed ACT-078573) is an orexin antagonist, functioning as a competitive receptor antagonist of the OX1 and OX2 orexin receptors, which was being developed by the pharmaceutical companies Actelion and GSK for the treatment of insomnia. Development of the drug was abandoned in January 2011 due to undisclosed issues pertaining to Almorexant's safety profile.

TCS-OX2-29 is an orexin antagonist. It was the first non-peptide antagonist developed that is selective for the orexin receptor subtype OX2, with an IC50 of 40nM and selectivity of around 250x for OX2 over OX1 receptors. Orexin antagonists are expected to be useful for the treatment of insomnia, with subtype-selective antagonists such as TCS-OX2-29 potentially offering more specificity of action compared to non-selective orexin antagonists like almorexant. TCS-OX2-29 Inhibits orexin A induced IP3 accumulation and ERK1/2 phosphorylation in CHO cells transfected with the OX2 receptor.
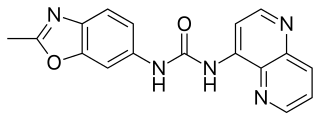
SB-334867 is an orexin antagonist. It was the first non-peptide antagonist developed that is selective for the orexin receptor subtype OX1, with around 50x selectivity for OX1 over OX2 receptors. It has been shown to produce sedative and anorectic effects in animals, and has been useful in characterising the orexinergic regulation of brain systems involved with appetite and sleep, as well as other physiological processes. The hydrochloride salt of SB-334867 has been demonstrated to be hydrolytically unstable, both in solution and as the solid. Orexin antagonists have multiple potential clinical applications including the treatment of drug addiction, insomnia, obesity and diabetes.
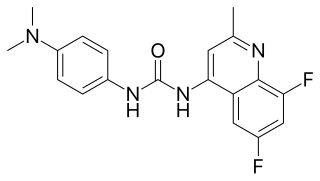
SB-408124 is a drug which is a non-peptide antagonist selective for the orexin receptor subtype OX1, with around 70x selectivity for OX1 over OX2 receptors, and improved oral bioavailability compared to the older OX1 antagonist SB-334867. It is used in scientific research into the function of orexinergic neurons in the body.
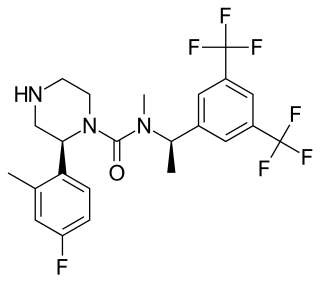
Vestipitant (INN) is a drug developed by GlaxoSmithKline which acts as a selective antagonist for the NK1 receptor. It is under development as a potential antiemetic and anxiolytic drug, and as a treatment for tinnitus and insomnia.

Nelotanserin is a drug developed by Arena Pharmaceuticals which acts as an inverse agonist on the serotonin receptor subtype 5-HT2A and was under development for the treatment of insomnia. It was shown to be effective and well-tolerated in clinical trials, but development was halted in December 2008 because the substance did not meet the trial's effectiveness endpoints. Research continues on newer analogues which may potentially be more successful. More recently, nelotanserin has been repurposed for the treatment of Lewy body disease. As of 2017, it is in phase II clinical trials for this indication.

Suvorexant, sold under the trade name Belsomra, is a medication for the treatment of insomnia. It is effective for insomnia, at least for four weeks and as compared to a placebo.
An orexin receptor antagonist is a drug that inhibits the effect of orexin by acting as a receptor antagonist of the orexin receptor. Potential applications include treatment of sleep disorders such as insomnia.
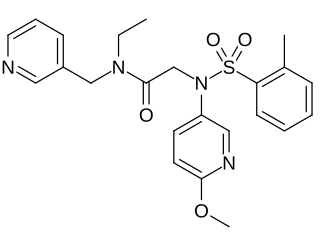
EMPA is a selective antagonist of the OX2 receptor, with 900-fold selectivity in binding for OX2 over OX1.

Filorexant (INN, USAN) (code name MK-6096) is an orexin antagonist which is or was under development by Merck for the treatment of insomnia. It is a dual antagonist of the OX1 and OX2 receptors. As of March 2014, filorexant has completed phase II clinical trials. It was also investigated as a migraine prophylaxis, but was not found effective, and in major depressive disorder and painful diabetic neuropathy. As of May 2015, filorexant is no longer listed on Merck's online development pipeline.

Seltorexant (former developmental code names MIN-202, JNJ-42847922, JNJ-922) is a selective, small-molecule antagonist of the OX2 receptor that is under development by Minerva Neurosciences and Johnson & Johnson's Janssen Pharmaceutica for the treatment of insomnia and major depressive disorder (MDD). As of December 2015, it is in phase II clinical trials for both insomnia and MDD.

Lemborexant (INN) (code name E-2006) is a dual antagonist of the orexin OX1 and OX2 receptors which is under development by Eisai for the treatment of insomnia. In June 2016, Eisai initiated phase 3 clinical trials which are currently underway in the US, France, Germany, Italy, Japan, Poland, Spain and the UK, and are expected to extend to Canada.


















