Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.

Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

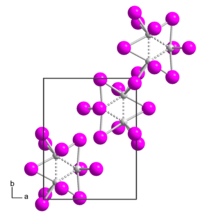
Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.
Rhenium(VII) sulfide is a chemical compound with the formula Re2S7. It has a complex structure, but can be synthesized from direct combination of the elements:
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions.
Samarium(III) iodide is an inorganic compound, a salt of samarium and hydroiodic acid with the chemical formula SmI
3.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.
Osmium iodide refers to compounds of osmium with the formula OsIn. Several have been mentioned in the literature, but all iodides except the tetraiodide have been verified by X-ray crystallography.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Cerium diiodide is an iodide of cerium, with the chemical formula of CeI2.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.
Ruthenium(III) iodide is a chemical compound containing ruthenium and iodine with the formula RuI3. It is a black solid.
Rhenium tetraiodide is a binary chemical compound of rhenium and iodide with the chemical formula ReI
4.