
Tin is a chemical element with the symbol Sn (from Latin: stannum) and atomic number 50. It is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, SnO2. Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table, thanks to its magic number of protons. It has two main allotropes: at room temperature, the stable allotrope is β-tin, a silvery-white, malleable metal, but at low temperatures it transforms into the less dense grey α-tin, which has the diamond cubic structure. Metallic tin does not easily oxidize in air.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation states of the oxide. Al2O3 is an example of an amphoteric oxide.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition. To a chemist, rust is considered an ill-defined material, described as hydrated ferric oxide.
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound with the formula SnCl4. It is a colourless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as spiritus fumans libavii.
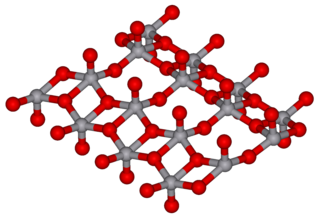
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4).

Tin(II) oxide is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.

Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.

Chromium(III) oxide is the inorganic compound of the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Tin(II) hydroxide, Sn(OH)2, also known as stannous hydroxide, is an inorganic compound tin(II). The only related material for which definitive information is available is the oxy hydroxide Sn6O4(OH)4, but other related materials are claimed. They are all white solids that are insoluble in water.
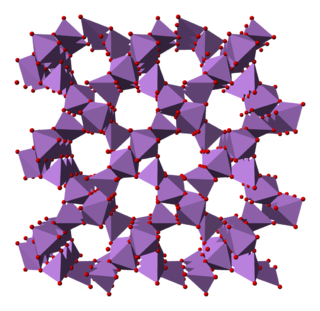
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All arsenic compounds are highly toxic and thus find only limited commercial applications.

Cadmium oxide is an inorganic compound with the formula CdO. It is one of the main precursors to other cadmium compounds. It crystallizes in a cubic rocksalt lattice like sodium chloride, with octahedral cation and anion centers. It occurs naturally as the rare mineral monteponite. Cadmium oxide can be found as a colorless amorphous powder or as brown or red crystals. Cadmium oxide is an n-type semiconductor with a band gap of 2.18 eV at room temperature.

Tin-glazing is the process of giving ceramic items a tin-based glaze that is white, glossy and opaque, which is normally applied to red or buff earthenware. The opacity and whiteness of tin glaze encourage its frequent decoration with overglaze colour. Majolica, delftware and faience are among the terms used for common types of tin-glazed pottery. An alternative is lead-glazing, where the basic glaze is transparent; some types of pottery use both.

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
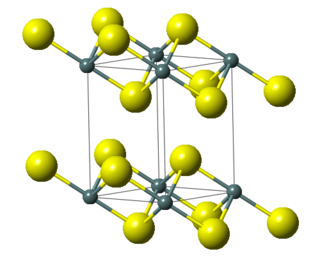
Tin(IV) sulfide is a compound with the formula SnS
2. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers. It occurs naturally as the rare mineral berndtite. It is useful as semiconductor material with band gap 2.2 eV.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.

Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the chemical formula H
3OBF
4.

Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relationship with aluminium.
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallises as a bright orange solid that dissolves readily in nonpolar solvents such as benzene.
Antimony sulfate, Sb2(SO4)3, is a hygroscopic material is formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.





















