In vitro fertilisation (IVF) is a process of fertilisation in which an egg is combined with sperm in vitro. The process involves monitoring and stimulating a woman's ovulatory process, then removing an ovum or ova from her ovaries and enabling a man's sperm to fertilise them in a culture medium in a laboratory. After a fertilised egg (zygote) undergoes embryo culture for 2–6 days, it is transferred by catheter into the uterus, with the intention of establishing a successful pregnancy.
Reproductive technology encompasses all current and anticipated uses of technology in human and animal reproduction, including assisted reproductive technology (ART), contraception and others. It is also termed Assisted Reproductive Technology, where it entails an array of appliances and procedures that enable the realization of safe, improved and healthier reproduction. While this is not true of all people, for an array of married couples, the ability to have children is vital. But through the technology, infertile couples have been provided with options that would allow them to conceive children.

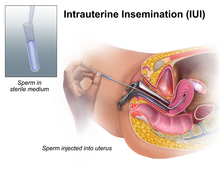
Artificial insemination is the deliberate introduction of sperm into a female's cervix or uterine cavity for the purpose of achieving a pregnancy through in vivo fertilization by means other than sexual intercourse. It is a fertility treatment for humans, and is a common practice in animal breeding, including dairy cattle and pigs.
Insemination is the introduction of sperm into a female or hermaphrodite's reproductive system in order to fertilize the ovum through sexual reproduction. The sperm enters into the uterus of a mammal or the oviduct of an oviparous (egg-laying) animal. Female humans and other mammals are inseminated during sexual intercourse or copulation, but can also be inseminated by artificial insemination.
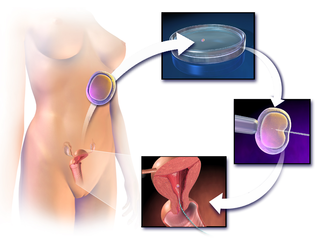
Assisted reproductive technology (ART) includes medical procedures used primarily to address infertility. This subject involves procedures such as in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), and cryopreservation of gametes and embryos, and the use of fertility medication. When used to address infertility, ART may also be referred to as fertility treatment. ART mainly belongs to the field of reproductive endocrinology and infertility. Some forms of ART may be used with regard to fertile couples for genetic purpose. ART may also be used in surrogacy arrangements, although not all surrogacy arrangements involve ART. The existence of sterility will not always require ART to be the first option to consider, as there are occasions when its cause is a mild disorder that can be solved with more conventional treatments or with behaviors based on promoting health and reproductive habits.
Embryo transfer refers to a step in the process of assisted reproduction in which embryos are placed into the uterus of a female with the intent to establish a pregnancy. This technique - which is often used in connection with in vitro fertilization (IVF) - may be used in humans or in other animals, in which situations and goals may vary.

Surrogacy is an arrangement, often supported by a legal agreement, whereby a woman agrees to pregnancy and childbirth on behalf of (an)other person(s) who will become the child's legal parent(s) after birth. People pursue surrogacy for a variety of reasons such as infertility, dangers or undesirable factors of pregnancy, or when pregnancy is a medical impossibility.
Third-party reproduction or donor-assisted reproduction is any human reproduction in which DNA or gestation is provided by a third party or donor other than the one or two parents who will raise the resulting child. This goes beyond the traditional father–mother model, and the third party's involvement is limited to the reproductive process and does not extend into the raising of the child. Third-party reproduction is used by couples unable to reproduce by traditional means, by same-sex couples, and by men and women without a partner. Where donor gametes are provided by a donor, the donor will be a biological parent of the resulting child, but in third party reproduction, he or she will not be the caring parent.
Egg donation is the process by which a woman donates eggs to enable another woman to conceive as part of an assisted reproduction treatment or for biomedical research. For assisted reproduction purposes, egg donation typically involves in vitro fertilization technology, with the eggs being fertilized in the laboratory; more rarely, unfertilized eggs may be frozen and stored for later use. Egg donation is a third-party reproduction as part of assisted reproductive technology.
Human sexual reproduction, to produce offspring, begins with fertilization. Successful reproduction typically involves sexual intercourse between a healthy, sexually mature and fertile male and female. During sexual intercourse, sperm cells are ejaculated into the vagina through the penis, resulting in fertilization of an ovum to form a zygote.

Fertility clinics are medical clinics that assist couples, and sometimes individuals, who want to become parents but for medical reasons have been unable to achieve this goal via the natural course. Clinics apply a number of diagnosis tests and sometimes very advanced medical treatments to achieve conceptions and pregnancies.

Oocyte cryopreservation is a procedure to preserve a woman's eggs (oocytes). The technique is often used to delay pregnancy. At the time pregnancy is desired, the eggs can be thawed, fertilized, and transferred to the uterus as embryos. Many studies have suggested infertility problems as germ cell deterioration related to aging. The procedure's success rate varies according to the woman's age, health, and genetic factors. The first human birth of oocyte cryopreservation was reported in 1986.
Fertility preservation is the effort to help cancer patients retain their fertility, or ability to procreate. Research into how cancer, ageing and other health conditions effect reproductive health and preservation options are growing. Specifically sparked in part by the increase in the survival rate of cancer patients.
Fertility tourism is the practice of traveling to another country or jurisdiction for fertility treatment, and may be regarded as a form of medical tourism. A person who can become pregnant is considered to have fertility issues if they are unable to have a clinical pregnancy after 12 months of unprotected intercourse. Infertility, or the inability to get pregnant, affects about 8-12% of couples looking to conceive or 186 million people globally. In some places, rates of infertility surpass the global average and can go up to 30% depending on the country. Areas with lack of resources, such as assisted reproductive technologies (ARTs), tend to correlate with the highest rates of infertility.
Pregnancy rate is the success rate for getting pregnant. It is the percentage of all attempts that leads to pregnancy, with attempts generally referring to menstrual cycles where insemination or any artificial equivalent is used, which may be simple artificial insemination (AI) or AI with additional in vitro fertilization (IVF).
A conception device is a medical device which is used to assist in the achievement of a pregnancy, often, but not always, by means other than sexual intercourse. This article deals exclusively with conception devices for human reproduction.
Religious response to assisted reproductive technology deals with the new challenges for traditional social and religious communities raised by modern assisted reproductive technology. Because many religious communities have strong opinions and religious legislation regarding marriage, sex and reproduction, modern fertility technology has forced religions to respond.
Partner-assisted reproduction, reception of oocytes from partner (ROPA), reciprocal IVF,shared motherhood, partner IVF or co-IVF is a method of family building that is used by couples who both possess female reproductive organs. The method uses in vitro fertilization (IVF), a method that means eggs are removed from the ovaries, fertilized in a laboratory, and then one or more of the resulting embryos are placed in the uterus to hopefully create a pregnancy. Reciprocal IVF differs from standard IVF in that two partners are involved: the eggs are taken from one partner, and the other partner carries the pregnancy. In this way, the process is mechanically identical to IVF with egg donation. Reciprocal IVF offers the highest chance for pregnancy and a lower chance of a multiple births.
Transgender pregnancy is the gestation of one or more embryos or fetuses by transgender people. This is possible for those born with female reproductive systems. However, transition-related treatments may impact fertility. Transgender men and nonbinary people who are or wish to become pregnant face social, medical, legal, and psychological concerns. As uterus transplantations are currently experimental, and none have successfully been performed on trans women, they cannot become pregnant.
Repeated implantation failure (RIF) is the repeated failure of the embryo to implant onto the side of the uterus wall following IVF treatment. Implantation happens at 6–7 days after conception and involves the embedding of the growing embryo into the mothers uterus and a connection being formed. A successful implantation can be determined by using an ultrasound to view the sac which the baby grows in, inside the uterus.