 | |
| Names | |
|---|---|
| IUPAC name Thulium(III) oxide | |
| Other names Thulium oxide, thulium sesquioxide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.670 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Tm2O3 | |
| Molar mass | 385.866 g/mol |
| Appearance | greenish-white cubic crystals |
| Density | 8.6 g/cm3 |
| Melting point | 2,341 °C (4,246 °F; 2,614 K) |
| Boiling point | 3,945 °C (7,133 °F; 4,218 K) |
| Solubility | Slightly soluble in acids |
| +51,444·10−6 cm3/mol | |
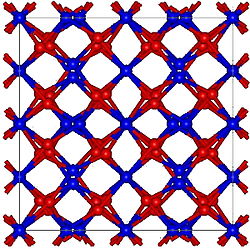
| Structure | |
| Cubic, cI80 [1] | |
| Ia-3, No. 206 [1] | |
Formula units (Z) | 16 [1] |
| Thermochemistry | |
Heat capacity (C) | 2.515 °Cp [2] (25 °C) |
| Hazards | |
| GHS labelling: | |
 | |
| Safety data sheet (SDS) | Sigma-Aldrich |
| Related compounds | |
Other anions | Thulium(III) chloride |
Other cations | Erbium(III) oxide Ytterbium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Thulium(III) oxide is a pale green crystalline compound, with the formula Tm 2 O 3. It was first isolated in 1879, from an impure sample of erbia, by Swedish chemist Per Teodor Cleve, who named it thulia.