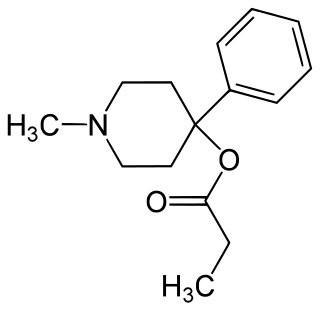
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it does not exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.

Phenazone is an analgesic, antipyretic and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative, trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin, aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.
Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.
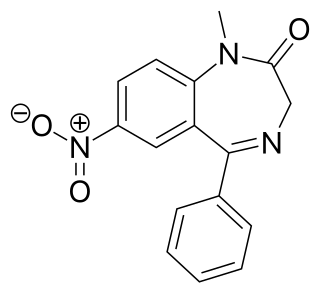
Nimetazepam is an intermediate-acting hypnotic drug which is a benzodiazepine derivative. It was first synthesized by a team at Hoffmann-La Roche in 1964. It possesses powerful hypnotic, anxiolytic, sedative, and skeletal muscle relaxant properties. Nimetazepam is also a particularly potent anticonvulsant. It is marketed in 5 mg tablets known as Erimin, which is the brand name manufactured and marketed by the large Japanese corporation Sumitomo. Japan is the sole manufacturer of nimetazepam in the world. Outside of Japan, Erimin is available in much of East and Southeast Asia and was widely prescribed for the short-term treatment of severe insomnia in patients who have difficulty falling asleep or maintaining sleep. Sumitomo has ceased manufacturing Erimin since November 2015. It is still available as a generic drug or as Lavol.

Adinazolam is a tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It possesses anxiolytic, anticonvulsant, sedative, and antidepressant properties. Adinazolam was developed by Jackson B. Hester, who was seeking to enhance the antidepressant properties of alprazolam, which he also developed. Adinazolam was never FDA approved and never made available to the public market; however, it has been sold as a designer drug.

Aminorex is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension. In the U.S., it is an illegal Schedule I drug, meaning it has high abuse potential, no accepted medical use, and a poor safety profile.
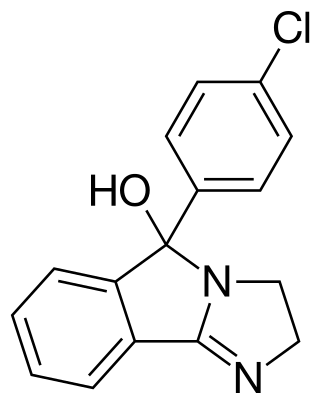
Mazindol, sold under the brand names Mazanor and Sanorex, is a central nervous system (CNS) stimulant which is used as an appetite suppressant. It was developed by Sandoz-Wander in the 1960s.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

Suritozole is an investigational cognition enhancer. It acts as a partial inverse agonist at the benzodiazepine receptor site on the GABAA ion channel complex, but does not have either anxiogenic or convulsant effects, unlike other BZD inverse agonists such as DMCM. It was investigated for the treatment of depression and Alzheimer's disease, but clinical development seems to have been discontinued.

Tametraline (CP-24,441) is the parent of a series of chemical compounds investigated at Pfizer that eventually led to the development of sertraline (CP-51,974-1).

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.

Flumezapine is an abandoned, investigational antipsychotic drug that was studied for the treatment of schizophrenia. Flumezapine failed clinical trials due to concern for liver and muscle toxicity. Flumezapine is structurally related to the common antipsychotic olanzapine—a point that was used against its manufacturer, Eli Lilly and Company, in a lawsuit in which generic manufacturers sought to void the patent on brand name olanzapine (Zyprexa). Although flumezapine does not differ greatly from olanzapine in terms of its structure, the difference was considered to be non-obvious, and Eli Lilly's patent rights on Zyprexa were upheld.
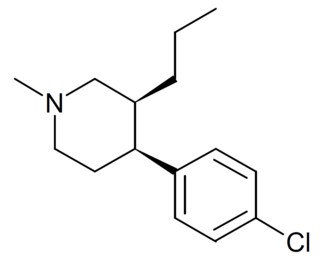
1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine is a drug developed by a team led by Alan Kozikowski, which acts as a potent dopamine reuptake inhibitor, and was developed as a potential therapeutic agent for the treatment of cocaine addiction. As with related compounds such as nocaine, it is a structurally simplified derivative of related phenyltropane compounds. Its activity at the serotonin and noradrenaline transporters has not been published, though most related 4-phenylpiperidine derivatives are relatively selective for inhibiting dopamine reuptake over the other monoamine neurotransmitters. While several of its isomers are active, the (3S,4S)-enantiomer is by far the most potent. The rearranged structural isomer 2-[1-(4-chlorophenyl)butyl]piperidine is also a potent inhibitor of dopamine reuptake.
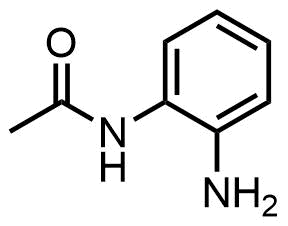
2-Aminoacetanilide is a chemical compound which is a amino derivative of acetanilide and ortho-isomer of aminoacetanilide. There are two other isomers of aminoacetanilide, 3-aminoacetanilide and 4-aminoacetanilide. Aminoacetanilide derivatives are important synthetic intermediates in heterocyclic and aromatic synthesis. These derivatives have found applications in pharmaceutical industry and dyes and pigment industry.
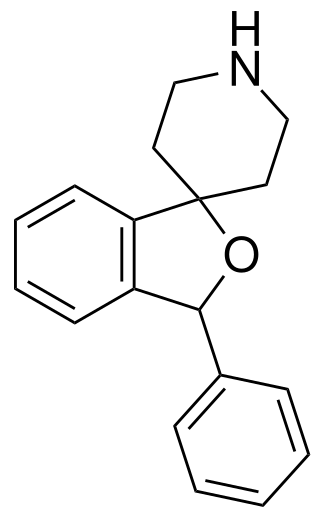
HP-505 is a triple reuptake inhibitor that was investigated by Hoechst-Roussel Pharmaceuticals. In mice, HP-505 was a potent inhibitor of tetrabenazine-induced ptosis which may indicate antidepressant activity.


















