
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1923, by Coster and Hevesy, making it the last stable element to be discovered. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.

The metal–oxide–semiconductor field-effect transistor, also known as the metal–oxide–silicon transistor, is a type of insulated-gate field-effect transistor that is fabricated by the controlled oxidation of a semiconductor, typically silicon. The voltage of the covered gate determines the electrical conductivity of the device; this ability to change conductivity with the amount of applied voltage can be used for amplifying or switching electronic signals.
Pyroelectricity is a property of certain crystals which are naturally electrically polarized and as a result contain large electric fields. Pyroelectricity can be described as the ability of certain materials to generate a temporary voltage when they are heated or cooled. The change in temperature modifies the positions of the atoms slightly within the crystal structure, such that the polarization of the material changes. This polarization change gives rise to a voltage across the crystal. If the temperature stays constant at its new value, the pyroelectric voltage gradually disappears due to leakage current. The leakage can be due to electrons moving through the crystal, ions moving through the air, or current leaking through a voltmeter attached across the crystal.

A thin-film transistor (TFT) is a special type of metal–oxide–semiconductor field-effect transistor (MOSFET) made by depositing thin films of an active semiconductor layer as well as the dielectric layer and metallic contacts over a supporting substrate. A common substrate is glass, because the primary application of TFTs is in liquid-crystal displays (LCDs). This differs from the conventional bulk MOSFET transistor, where the semiconductor material typically is the substrate, such as a silicon wafer.

Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride.

Yttrium oxide, also known as yttria, is Y2O3. It is an air-stable, white solid substance.
The term high-κ dielectric refers to a material with a high dielectric constant, as compared to silicon dioxide. High-κ dielectrics are used in semiconductor manufacturing processes where they are usually used to replace a silicon dioxide gate dielectric or another dielectric layer of a device. The implementation of high-κ gate dielectrics is one of several strategies developed to allow further miniaturization of microelectronic components, colloquially referred to as extending Moore's Law. Sometimes, these materials are called "high-k", instead of "high-κ".

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
Atomic layer deposition (ALD) is a thin-film deposition technique based on the sequential use of a gas phase chemical process; it is a subclass of chemical vapour deposition. The majority of ALD reactions use two chemicals called precursors. These precursors react with the surface of a material one at a time in a sequential, self-limiting, manner. Through the repeated exposure to separate precursors, a thin film is slowly deposited. ALD is a key process in the fabrication of semiconductor devices, and part of the set of tools available for the synthesis of nanomaterials.

Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula Ta
2O
5. It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. Ta
2O
5 is an inert material with a high refractive index and low absorption, which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant.

Vanadium(IV) oxide or vanadium dioxide is an inorganic compound with the formula VO2. It is a dark blue solid. Vanadium(IV) dioxide is amphoteric, dissolving in non-oxidising acids to give the blue vanadyl ion, [VO]2+ and in alkali to give the brown [V4O9]2− ion, or at high pH [VO4]4−. VO2 has a phase transition very close to room temperature (~66 °C). Electrical resistivity, opacity, etc, can change up several orders. Owing to these properties, it has been used in surface coating, sensors, and imaging. Potential applications include use in memory devices, phase-change switches, aerospace communication systems and neuromorphic computing.
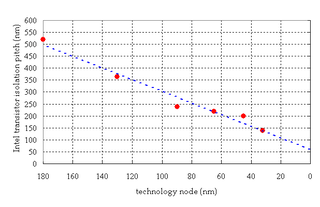
Shallow trench isolation (STI), also known as box isolation technique, is an integrated circuit feature which prevents electric current leakage between adjacent semiconductor device components. STI is generally used on CMOS process technology nodes of 250 nanometers and smaller. Older CMOS technologies and non-MOS technologies commonly use isolation based on LOCOS.
Bismuth ferrite (BiFeO3, also commonly referred to as BFO in materials science) is an inorganic chemical compound with perovskite structure and one of the most promising multiferroic materials. The room-temperature phase of BiFeO3 is classed as rhombohedral belonging to the space group R3c. It is synthesized in bulk and thin film form and both its antiferromagnetic (G type ordering) Néel temperature (approximately 653 K) and ferroelectric Curie temperature are well above room temperature (approximately 1100K). Ferroelectric polarization occurs along the pseudocubic direction () with a magnitude of 90–95 μC/cm2.
Resistive random-access memory is a type of non-volatile (NV) random-access (RAM) computer memory that works by changing the resistance across a dielectric solid-state material, often referred to as a memristor. This technology bears some similarities to conductive-bridging RAM (CBRAM), and phase-change memory (PCM).
Scanning capacitance microscopy (SCM) is a variety of scanning probe microscopy in which a narrow probe electrode is positioned in contact or close proximity of a sample's surface and scanned. SCM characterizes the surface of the sample using information obtained from the change in electrostatic capacitance between the surface and the probe.
Hafnium silicate is the hafnium(IV) salt of silicic acid with the chemical formula of HfSiO4.
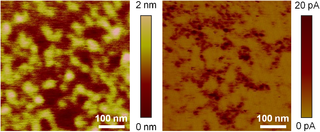
Conductive atomic force microscopy (C-AFM) or current sensing atomic force microscopy (CS-AFM) is a mode in atomic force microscopy (AFM) that simultaneously measures the topography of a material and the electric current flow at the contact point of the tip with the surface of the sample. The topography is measured by detecting the deflection of the cantilever using an optical system, while the current is detected using a current-to-voltage preamplifier. The fact that the CAFM uses two different detection systems is a strong advantage compared to scanning tunneling microscopy (STM). Basically, in STM the topography picture is constructed based on the current flowing between the tip and the sample. Therefore, when a portion of a sample is scanned with an STM, it is not possible to discern if the current fluctuations are related to a change in the topography or to a change in the sample conductivity.
Flexible silicon refers to a flexible piece of mono-crystalline silicon. Several processes have been demonstrated in the literature for obtaining flexible silicon from single crystal silicon wafers.
A ferroelectric field-effect transistor is a type of field-effect transistor that includes a ferroelectric material sandwiched between the gate electrode and source-drain conduction region of the device. Permanent electrical field polarisation in the ferroelectric causes this type of device to retain the transistor's state in the absence of any electrical bias.












