Related Research Articles

Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.

Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat advanced prostate cancer. To a lesser extent, it is used for early prostate cancer at a higher dosage as a monotherapy. Bicalutamide is also used to treat excessive hair growth in women, as a component of feminizing hormone therapy for transgender women, to treat early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.
Fulvestrant, sold under the brand name Faslodex among others, is a medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with palbociclib in women with disease progression after endocrine therapy. It is given by injection into a muscle.

Drostanolone propionate, or dromostanolone propionate, sold under the brand names Drolban, Masteril, and Masteron among others, is an androgen and anabolic steroid (AAS) medication which was used to treat breast cancer in women but is now no longer marketed. It is given by injection into muscle.

Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
This article is about the discovery and development of antiandrogens, or androgen receptor (AR) antagonists.
EPI-001 is the first inhibitor of the androgen receptor amino-terminal domain. The single stereoisomer of EPI-001, EPI-002, is a first-in-class drug that the USAN council assigned a new stem class "-aniten" and the generic name "ralaniten". This distinguishes the anitens novel molecular mechanism from anti androgens that bind the C-terminus ligand-binding domain and have the stem class "lutamide". EPI-001 and its stereoisomers and analogues were discovered by Dr. Marianne Sadar and Dr. Raymond Andersen who co-founded the pharmaceutical company ESSA Pharma Inc for the clinical development of anitens for the treatment of castration-resistant prostate cancer (CRPC).

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.

Topilutamide, known more commonly as fluridil and sold under the brand name Eucapil, is an antiandrogen medication which is used in the treatment of pattern hair loss in men and women. It is used as a topical medication and is applied to the scalp. Topilutamide belongs to a class of molecules known as perfluoroacylamido-arylpropanamides.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.
A selective estrogen receptor degrader or downregulator (SERD) is a type of drug which binds to the estrogen receptor (ER) and, in the process of doing so, causes the ER to be degraded and thus downregulated. They are used to treat estrogen receptor-sensitive or progesterone receptor-sensitive breast cancer, along with older classes of drugs like selective estrogen receptor modulators (SERMs) and aromatase inhibitors.

Ralaniten acetate is a first-in-class antiandrogen that targets the N-terminal domain (NTD) of the androgen receptor (AR) developed by ESSA Pharmaceuticals and was under investigation for the treatment of prostate cancer. This mechanism of action is believed to allow the drug to block signaling from the AR and its splice variants. EPI-506 is a derivative of bisphenol A and a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001, and was developed as a successor of EPI-001. The drug reached phase I/II prior to the discontinuation of its development. It showed signs of efficacy in the form of prostatic specific antigen (PSA) decreases (4–29%) predominantly at higher doses (≥1,280 mg) in some patients but also caused side effects and was discontinued by its developer in favor of next-generation AR NTD inhibitors with improved potency and tolerability.
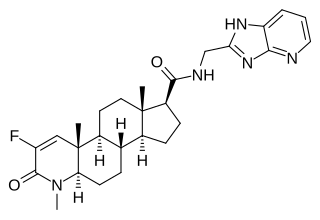
MK-0773, also known as PF-05314882, is a steroidal, orally active selective androgen receptor modulator (SARM) that was under development by Merck and GTx for the treatment of sarcopenia in women and men. Clinical trials for sarcopenia began in late 2007 but the collaboration between Merck and GTx ended in early 2010 and GTx terminated development of MK-0773 shortly thereafter.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.
Dimethylcurcumin is a nonsteroidal antiandrogen and a synthetic curcuminoid which is under development by AndroScience Corporation as a topical medication for the treatment of acne vulgaris. It has also been under investigation for the treatment of male pattern hair loss, spinal muscular atrophy, and wounds, but no development has been reported for these indications. There has been interest in the drug for the potential treatment of prostate cancer as well. As of 2017, it is in phase II clinical trials for acne vulgaris.

BMS-641988 is a nonsteroidal antiandrogen which was developed by Bristol-Myers Squibb for the treatment of prostate cancer but was never marketed. It acts as a potent competitive antagonist of the androgen receptor (AR) (Ki = 10 nM; IC50 = 56 nM). The drug was found to have 20-fold higher affinity for the AR than bicalutamide in MDA-MB-453 cells, and showed 3- to 7-fold the antiandrogenic activity of bicalutamide in vitro. It may have some weak partial agonist activity at the androgen receptor. BMS-641988 is transformed by CYP3A4 into BMS-570511, and this metabolite is then reduced to BMS-501949 by cytosolic reductases. All three compounds show similar antiandrogenic activity. In addition to its antiandrogenic activity, BMS-641988 shows activity as a negative allosteric modulator of the GABAA receptor, and can produce seizures in animals at sufficiently high doses. It also shows some drug-induced QT prolongation. BMS-641988 reached phase I clinical trials prior to the discontinuation of its development. The clinical development of BMS-641988 was terminated due to the occurrence of a seizure in a patient during a phase I study.

EM-5854 is a steroidal antiandrogen which was under development by Endoceutics, Inc. for the treatment of prostate cancer. It was first described in a patent in 2008, and was further characterized in 2012. EM-5854 reached phase I/II clinical trials for the treatment of prostate cancer but development was discontinued in March 2019.
EPI-7386 is an N-terminal domain antiandrogen, or antagonist of the N-terminal domain (NTD) of the androgen receptor (AR), which is under development for the treatment of prostate cancer. The compound was developed as a successor of previous drugs in the EPI series such as EPI-001, ralaniten (EPI-002), and ralaniten acetate (EPI-506). EPI-7386 shows 20-fold higher antiandrogenic potency than ralaniten in vitro (IC50 = 535 nM vs. 9,580 nM, respectively), as well as greater stability in human hepatocytes. It is planned to enter phase I clinical trials in 2020.
References
- 1 2 Lai, AC; Crews, CM (25 November 2016). "Induced protein degradation: an emerging drug discovery paradigm". Nature Reviews. Drug Discovery. 16 (2): 101–114. doi:10.1038/nrd.2016.211. PMC 5684876 . PMID 27885283.
- ↑ "ASCJ 9 - AdisInsight".