Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.
Vanadium tetrachloride is the inorganic compound with the formula VCl4. This reddish-brown liquid serves as a useful reagent for the preparation of other vanadium compounds.

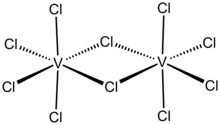
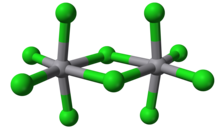
Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Platinum(II) chloride describes the inorganic compounds with the formula PtCl2. They are precursor used in the preparation of other platinum compounds. Platinum(II) chloride exists in two crystalline forms (polymorphs), but the main properties are somewhat similar: dark brown, insoluble in water, diamagnetic, and odorless.

Platinum(IV) chloride is the inorganic compound of platinum and chlorine with the empirical formula PtCl4. This brown solid features platinum in the 4+ oxidation state.
Vanadium chloride may refer to:

Thorium(IV) chloride describes a family of inorganic compounds with the formula ThCl4(H2O)n. Both the anhydrous and tetrahydrate (n = 4) forms are known. They are hygroscopic, water-soluble white salts.
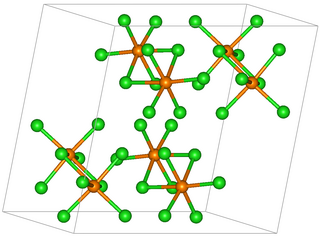
Rhenium pentachloride is an inorganic compound with the formula Re2Cl10. This red-brown solid is paramagnetic.

Osmium(IV) chloride or osmium tetrachloride is the inorganic compound composed of osmium and chlorine with the empirical formula OsCl4. It exists in two polymorphs (crystalline forms). The compound is used to prepare other osmium complexes.
A pentachloride is a compound or ion that contains five chlorine atoms or ions. Common pentachlorides include:

Trithiazyl trichloride is the inorganic compound with the formula (NSCl)3. A white solid, it is a precursor to other sulfur nitrides, but has no commercial applications.
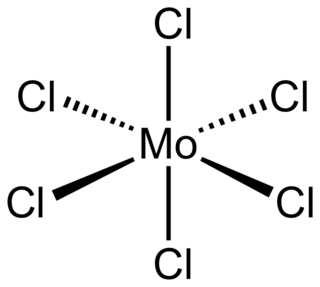
Molybdenum(VI) chloride is the inorganic compound with the formula MoCl6. It is a black diamagnetic solid. The molecules adopt an octahedral structure as seen in β-tungsten(VI) chloride.

Rhenium(VI) chloride is the inorganic compound with the formula ReCl6. It is a black paramagnetic solid. The molecules adopt an octahedral structure as seen in tungsten(VI) chloride.

Vanadium (V) chloride chlorimide is a chemical compound containing vanadium in a +5 oxidation state bound to three chlorine atoms and with a double bond to a chlorimide group (=NCl). It has formula VNCl4. This can be also considered as a chloroiminato complex.

Protactinium(IV) chloride is an inorganic compound. It is an actinide halide, a salt composed of protactinium and chlorine. It is radioactive, and has the chemical formula of PaCl4. It is a chartreuse-coloured (yellowish-green) crystal of the tetragonal crystal system.