
Dextromoramide is a powerful opioid analgesic approximately three times more potent than morphine but shorter acting. It is subject to drug prohibition regimes, both internationally through UN treaties and by the criminal law of individual nations, and is usually prescribed only in the Netherlands.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.

Phenoperidine, is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.

Metopon is an opioid analogue that is a methylated derivative of hydromorphone which was invented in 1929 as an analgesic.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.

Diethylthiambutene is an opioid analgesic drug developed in the 1950s which was mainly used as an anesthetic in veterinary medicine and continues, along with the other two thiambutenes dimethylthiambutene and ethylmethylthiambutene to be used for this purpose, particularly in Japan. It is now under international control under Schedule I of the UN Single Convention On Narcotic Drugs 1961, presumably due to high abuse potential, although little more information is available. It is listed under Schedule I of the US Controlled Substances Act as a Narcotic and has an ACSCN of 9616 with zero annual manufacturing quota as of 2013.

Dimethylthiambutene (N,N-Dimethyl-1-methyl-3,3-di-2-thienylallylamine, DMTB, trade names Ohton, Aminobutene, Dimethibutin, Kobaton, Takaton, Dimethibutin) is an opioid analgesic drug, most often used in veterinary medicine in Japan and to a lesser extent in other countries in the region and around the world. It is the most prominent and widely used of the thiambutenes, a series of open-chain opioids structurally related to methadone which are also called the thienyl derivative opioids which also includes diethylthiambutene and ethylmethylthiambutene, as well as the non-opioid cough suppressant tipepidine.

Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.
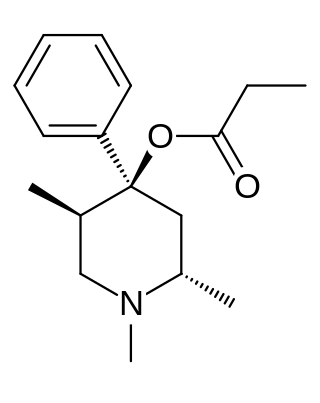
Trimeperidine (Promedol) is an opioid analgesic that is an analogue of prodine. It was developed in the early 1950s in the USSR during research into the related drug pethidine.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Phenampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and diampromide. It was invented in the 1960s by American Cyanamid Co. Although never given a general release, it was trialled and 50 mg codeine ≈ 60 mg phenampromide. Tests on the 2 isomers showed that all of the analgesic effects were caused by the (S) isomer. Introduction of a phenyl group to the 4-position of the piperidine-ring produces a drug 60-fold more potent than morphine. The most potent reported derivative is 4-hydroxy-4-phenyl phenapromide which displays analgesic activity some x150 greater than morphine.

Benzethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine.
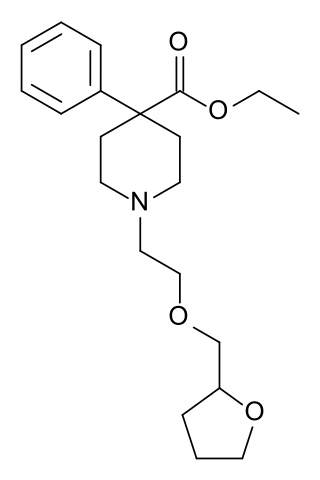
Furethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine), but with around 25x higher potency. According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine.

Morpheridine (Morpholinoethylnorpethidine) is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine). It is a strong analgesic with around 4 times the potency of pethidine, and unlike pethidine, does not cause convulsions, although it produces the standard opioid side effects such as sedation and respiratory depression.

The Thiambutenes are a family of opioid analgesic drugs developed at the British research laboratory of Burroughs-Wellcome in the late 1940s. The parent compound thiambutene has no analgesic effects, but several compounds from this group are analgesics with around the same potency as morphine.

Piperidylthiambutene (Piperidinohton) is a synthetic opioid analgesic drug from the thiambutene family, which has around the same potency as morphine. Piperidylthiambutene is structurally distinct from fentanyl, its analogues, and other synthetic opioids previously reported. If sold or obtained for the purpose of human consumption it could be considered a controlled substance analogue in some countries such as the US, Australia and New Zealand. Piperidylthiambutene has been sold as a designer drug, first appearing in late 2018.

Pyrrolidinylthiambutene is an opioid analgesic drug from the thiambutene family with around 3/4 of the potency of morphine. It would be considered an illegal controlled substance analogue in some countries such as the US, Australia and New Zealand, but is legal in countries not possessing a controlled-substances-analog-act equivalent.

Isomethadone (INN, BAN; trade name Liden; also known as isoamidone) is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of the two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide (HBr, free base conversion ratio 0.793), hydrochloride (HCl, 0.894), and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.

Noracymethadol (INN) is a synthetic opioid analgesic related to methadone that was never marketed. In a clinical trial of postpartum patients it was reported to produce analgesia comparable to that of morphine but with less nausea, dizziness, and drowsiness. Other side effects included salivation, ataxia, and respiratory depression that was reversible by naloxone. Similarly to many of its analogues, noracymethadol is a Schedule I controlled substance in the United States with an ACSCN of 9633 and 2013 annual manufacturing quota of 12 grammes. and is also controlled internationally under the United Nations Single Convention on Narcotic Drugs of 1961. The salts known are the gluconate and hydrochloride (0.903).




















