
Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron deficiency anemia.
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the SLC40A1 gene, and is part of the Ferroportin (Fpn)Family. Ferroportin is a transmembrane protein that transports iron from the inside of a cell to the outside of the cell. Ferroportin is the only known iron exporter.
Magnesium transporters are proteins that transport magnesium across the cell membrane. All forms of life require magnesium, yet the molecular mechanisms of Mg2+ uptake from the environment and the distribution of this vital element within the organism are only slowly being elucidated.
Glucose transporter 1, also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1), is a uniporter protein that in humans is encoded by the SLC2A1 gene. GLUT1 facilitates the transport of glucose across the plasma membranes of mammalian cells. This gene encodes a major glucose transporter in the mammalian blood-brain barrier. The encoded protein is found primarily in the cell membrane and on the cell surface, where it can also function as a receptor for human T-cell leukemia virus (HTLV) I and II. One good source of GLUT1 is erythrocyte membranes. GLUT1 accounts for 2 percent of the protein in the plasma membrane of erythrocytes. GLUT1, found in the plasma membrane of erythrocytes, is a classic example of a uniporter. After glucose is transported into the erythrocyte, it is rapidly phosphorylated, forming glucose-6-phosphate, which cannot leave the cell. Mutations in this gene can cause GLUT1 deficiency syndrome 1, GLUT1 deficiency syndrome 2, idiopathic generalized epilepsy 12, dystonia 9, and stomatin-deficient cryohydrocytosis.

SLC22A5 is a membrane transport protein associated with primary carnitine deficiency. This protein is involved in the active cellular uptake of carnitine. It acts a symporter, moving sodium ions and other organic cations across the membrane along with carnitine. Such polyspecific organic cation transporters in the liver, kidney, intestine, and other organs are critical for the elimination of many endogenous small organic cations as well as a wide array of drugs and environmental toxins. Mutations in the SLC22A5 gene cause systemic primary carnitine deficiency, which can lead to heart failure.

Thiamine transporter 2 (ThTr-2), also known as solute carrier family 19 member 3, is a protein that in humans is encoded by the SLC19A3 gene. SLC19A3 is a thiamine transporter.

Sodium/glucose cotransporter 1 (SGLT1) also known as solute carrier family 5 member 1 is a protein in humans that is encoded by the SLC5A1 gene which encodes the production of the SGLT1 protein to line the absorptive cells in the small intestine and the epithelial cells of the kidney tubules of the nephron for the purpose of glucose uptake into cells. Through the use of the sodium glucose cotransporter 1 protein, cells are able to obtain glucose which is further utilized to make and store energy for the cell.

Natural resistance-associated macrophage protein 1 is a protein that in humans is encoded by the SLC11A1 gene.

Solute carrier family 22, member 4, also known as SLC22A4, is a human gene; the encoded protein is known as the ergothioneine transporter.

Excitatory amino acid transporter 3 (EAAT3), is a protein that in humans is encoded by the SLC1A1 gene.

Solute carrier family 22 member 8, or organic anion transporter 3 (OAT3), is a protein that in humans is encoded by the SLC22A8 gene.

Solute carrier organic anion transporter family member 1A2 is a protein that in humans is encoded by the SLCO1A2 gene.

Mitoferrin-1 (Mfrn1) is a 38 kDa protein that is encoded by the SLC25A37 gene in humans. It is a member of the Mitochondrial carrier (MC) Superfamily, however, its metal cargo makes it distinct from other members of this family. Mfrn1 plays a key role in mitochondrial iron homeostasis as an iron transporter, importing ferrous iron from the intermembrane space of the mitochondria to the mitochondrial matrix for the biosynthesis of heme groups and Fe-S clusters. This process is tightly regulated, given the redox potential of Mitoferrin's iron cargo. Mfrn1 is paralogous to Mitoferrin-2 (Mfrn2), a 39 kDa protein encoded by the SLC25A28 gene in humans. Mfrn1 is highly expressed in differentiating erythroid cells and in other tissues at low levels, while Mfrn2 is expressed ubiquitously in non-erythroid tissues.

The high-affinity choline transporter (ChT) also known as solute carrier family 5 member 7 is a protein in humans that is encoded by the SLC5A7 gene. It is a cell membrane transporter and carries choline into acetylcholine-synthesizing neurons.

In molecular biology, the iron dependent repressors are a family of bacterial and archaeal transcriptional repressors.

In molecular biology, the ferric uptake regulator family is a family of bacterial proteins involved in regulating metal ion uptake and in metal homeostasis. The family is named for its founding member, known as the ferric uptake regulator or ferric uptake regulatory protein (Fur). Fur proteins are responsible for controlling the intracellular concentration of iron in many bacteria. Iron is essential for most organisms, but its concentration must be carefully managed over a wide range of environmental conditions; high concentrations can be toxic due to the formation of reactive oxygen species.
Cation diffusion facilitators (CDFs) are transmembrane proteins that provide tolerance of cells to divalent metal ions, such as cadmium, zinc, and cobalt. These proteins are considered to be efflux pumps that remove these divalent metal ions from cells. However, some members of the CDF superfamily are implicated in ion uptake. All members of the CDF family possess six putative transmembrane spanners with strongest conservation in the four N-terminal spanners. The Cation Diffusion Facilitator (CDF) Superfamily includes the following families:
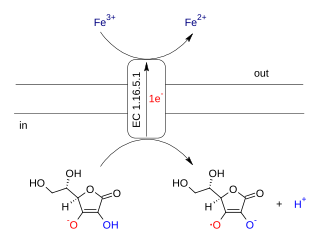
Ascorbate ferrireductase (transmembrane) is an enzyme with systematic name Fe(III):ascorbate oxidorectuctase (electron-translocating). This enzyme catalyses the following chemical reaction

Natural resistance-associated macrophage proteins (Nramps), also known as metal ion (Mn2+-iron) transporters (TC# 2.A.55), are a family of metal transport proteins found throughout all domains of life. Taking on an eleven-helix LeuT fold, the Nramp family is a member of the large APC Superfamily of secondary carriers. They transport a variety of transition metals such as manganese, cadmium, and manganese using an alternating access mechanism characteristic of secondary transporters.

Zinc transporter ZIP9, also known as Zrt- and Irt-like protein 9 (ZIP9) and solute carrier family 39 member 9, is a protein that in humans is encoded by the SLC39A9 gene. This protein is the 9th member out of 14 ZIP family proteins, which is a membrane androgen receptor (mAR) coupled to G proteins, and also classified as a zinc transporter protein. ZIP family proteins transport zinc metal from the extracellular environment into cells through cell membrane.



















