 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.666 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Rh2O3 | |
| Molar mass | 253.8092 g/mol |
| Appearance | dark grey odorless powder |
| Density | 8.20 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) (decomposes) |
| insoluble | |
| Solubility | insoluble in aqua regia |
| +104.0·10−6 cm3/mol | |
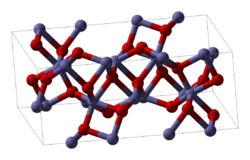
| Structure [1] | |
| hexagonal (corundum) | |
| R3c | |
a = 512.7 pm (hexagonal setting), c = 1385.3 pm (hexagonal setting) | |
| Hazards | |
| GHS labelling: [2] | |
  | |
| Danger | |
| H302+H332, H315, H319, H335 | |
| P280, P301+P330+P331, P302+P352, P304+P340, P312, P332+P313, P337+P313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Rhodium(III) oxide (or Rhodium sesquioxide) is the inorganic compound with the formula Rh2 O3. It is a gray solid that is insoluble in ordinary solvents.