Inhibitors of hormone synthesis


One effective strategy for starving tumor cells of growth- and survival-promoting hormones is to use drugs which inhibit the production of those hormones in their organ of origin.
Aromatase inhibitors
Aromatase inhibitors are an important class of drugs used for the treatment of breast cancer in postmenopausal women. At menopause, estrogen production in the ovaries ceases, but other tissues continue to produce estrogen through the action of the enzyme aromatase on androgens produced by the adrenal glands. When the action of aromatase is blocked, estrogen levels in post-menopausal women can drop to extremely low levels, causing growth arrest and/or apoptosis of hormone-responsive cancer cells.[ citation needed ]
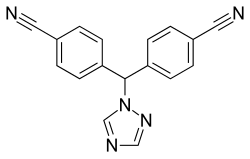
Letrozole and anastrozole are aromatase inhibitors which have been shown to be superior to tamoxifen for the first-line treatment of breast cancer in postmenopausal women. [1] Exemestane is an irreversible "aromatase inactivator" which is superior to megestrol acetate for treatment of tamoxifen-refractory metastatic breast cancer, and does not appear to have the osteoporosis-promoting side effects of other drugs in this class. [1]
Aminoglutethimide inhibits both aromatase and other enzymes critical for steroid hormone synthesis in the adrenal glands. It was formerly used for breast cancer treatment, but has since been replaced by more selective aromatase inhibitors. It can also be used for the treatment of hyperadrenocortical syndromes, such as Cushing's syndrome and hyperaldosteronism in adrenocortical carcinoma. [1]
GnRH analogues
Analogs of gonadotropin-releasing hormone (GnRH) can be used to induce a chemical castration, that is, complete suppression of the production of estrogen and progesterone from the female ovaries, or complete suppression of testosterone production from the male testes. This is due to a negative feedback effect of continuous stimulation of the pituitary gland by these hormones. Leuprorelin and goserelin are GnRH analogs which are used primarily for the treatment of hormone-responsive prostate cancer. Because the initial endocrine response to GnRH analogs is actually hypersecretion of gonadal steroids, hormone receptor antagonists such as flutamide are typically used to prevent a transient boost in tumor growth. [1]

