Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical nerve growth factor (NGF), a family which also includes NT-3 and NT-4/NT-5. Neurotrophic factors are found in the brain and the periphery. BDNF was first isolated from a pig brain in 1982 by Yves-Alain Barde and Hans Thoenen.

Neurotrophins are a family of proteins that induce the survival, development, and function of neurons.

Nerve growth factor (NGF) is a neurotrophic factor and neuropeptide primarily involved in the regulation of growth, maintenance, proliferation, and survival of certain target neurons. It is perhaps the prototypical growth factor, in that it was one of the first to be described. Since it was first isolated by Nobel Laureates Rita Levi-Montalcini and Stanley Cohen in 1956, numerous biological processes involving NGF have been identified, two of them being the survival of pancreatic beta cells and the regulation of the immune system.

Tyrosine-protein kinase ABL1 also known as ABL1 is a protein that, in humans, is encoded by the ABL1 gene located on chromosome 9. c-Abl is sometimes used to refer to the version of the gene found within the mammalian genome, while v-Abl refers to the viral gene, which was initially isolated from the Abelson murine leukemia virus.

Tropomyosin receptor kinase B (TrkB), also known as tyrosine receptor kinase B, or BDNF/NT-3 growth factors receptor or neurotrophic tyrosine kinase, receptor, type 2 is a protein that in humans is encoded by the NTRK2 gene. TrkB is a receptor for brain-derived neurotrophic factor (BDNF). The standard pronunciation for this protein is "track bee".

The p75 neurotrophin receptor (p75NTR) was first identified in 1973 as the low-affinity nerve growth factor receptor (LNGFR) before discovery that p75NTR bound other neurotrophins equally well as nerve growth factor. p75NTR is a neurotrophic factor receptor. Neurotrophic factor receptors bind Neurotrophins including Nerve growth factor, Neurotrophin-3, Brain-derived neurotrophic factor, and Neurotrophin-4. All neurotrophins bind to p75NTR. This also includes the immature pro-neurotrophin forms. Neurotrophic factor receptors, including p75NTR, are responsible for ensuring a proper density to target ratio of developing neurons, refining broader maps in development into precise connections. p75NTR is involved in pathways that promote neuronal survival and neuronal death.
Tropomyosin receptor kinase C (TrkC), also known as NT-3 growth factor receptor, neurotrophic tyrosine kinase receptor type 3, or TrkC tyrosine kinase is a protein that in humans is encoded by the NTRK3 gene.
Neurotrophic factors (NTFs) are a family of biomolecules – nearly all of which are peptides or small proteins – that support the growth, survival, and differentiation of both developing and mature neurons. Most NTFs exert their trophic effects on neurons by signaling through tyrosine kinases, usually a receptor tyrosine kinase. In the mature nervous system, they promote neuronal survival, induce synaptic plasticity, and modulate the formation of long-term memories. Neurotrophic factors also promote the initial growth and development of neurons in the central nervous system and peripheral nervous system, and they are capable of regrowing damaged neurons in test tubes and animal models. Some neurotrophic factors are also released by the target tissue in order to guide the growth of developing axons. Most neurotrophic factors belong to one of three families: (1) neurotrophins, (2) glial cell-line derived neurotrophic factor family ligands (GFLs), and (3) neuropoietic cytokines. Each family has its own distinct cell signaling mechanisms, although the cellular responses elicited often do overlap.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. The receptors are generally activated by dimerization and substrate presentation. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.

Neurotrophin-3 is a protein that in humans is encoded by the NTF3 gene.

Neurotrophin-4 (NT-4), also known as neurotrophin-5 (NT-5), is a protein that in humans is encoded by the NTF4 gene. It is a neurotrophic factor that signals predominantly through the TrkB receptor tyrosine kinase. NT-4 was first discovered and isolated from xenopus and viper in the year 1991 by Finn Hallbook et.al

Fibroblast growth factor receptor substrate 2 is a protein that in humans is encoded by the FRS2 gene.
Trk receptors are a family of tyrosine kinases that regulates synaptic strength and plasticity in the mammalian nervous system. Trk receptors affect neuronal survival and differentiation through several signaling cascades. However, the activation of these receptors also has significant effects on functional properties of neurons.

SHC-transforming protein 3 is a protein that in humans is encoded by the SHC3 gene.

Proto-oncogene tyrosine-protein kinase ROS is an enzyme that in humans is encoded by the ROS1 gene.
Neurotrophic factor receptors or neurotrophin receptors are a group of growth factor receptors which specifically bind to neurotrophins.
ETV6-NTRK3 gene fusion is the translocation of genetic material between the ETV6 gene located on the short arm of chromosome 12 at position p13.2 and the NTRK3 gene located on the long arm of chromosome 15 at position q25.3 to create the (12;15)(p13;q25) fusion gene, ETV6-NTRK3. This new gene consists of the 5' end of ETV6 fused to the 3' end of NTRK3. ETV6-NTRK3 therefore codes for a chimeric oncoprotein consisting of the helix-loop-helix (HLH) protein dimerization domain of the ETV6 protein fused to the tyrosine kinase domain of the NTRK3 protein. The ETV6 gene codes for the transcription factor protein, ETV6, which suppresses the expression of, and thereby regulates, various genes that in mice are required for normal hematopoiesis as well as the development and maintenance of the vascular network. NTRK3 codes for Tropomyosin receptor kinase C a NT-3 growth factor receptor cell surface protein that when bound to its growth factor ligand, neurotrophin-3, becomes an active tyrosine kinase that phosphorylates tyrosine residues on, and thereby stimulates, signaling proteins that promote the growth, survival, and proliferation of their parent cells. The tyrosine kinase of the ETV6-NTRK3 fusion protein is dysfunctional in that it is continuously active in phosphorylating tyrosine residues on, and thereby continuously stimulating, proteins that promote the growth, survival, and proliferation of their parent cells. In consequence, these cells take on malignant characteristics and are on the pathway of becoming cancerous. Indeed, the ETV6-NTRK3 fusion gene appears to be a critical driver of several types of cancers. It was originally identified in congenital fibrosarcoma and subsequently found in mammary secretory carcinoma, mammary analogue secretory carcinoma of salivary glands, salivary gland–type carcinoma of the thyroid, secretory carcinoma of the skin, congenital fibrosarcoma, congenital mesoblastic nephroma, rare cases of acute myelogenous leukemia, ALK-negative Inflammatory myofibroblastic tumour, cholangiocarcinoma, and radiation-induced papillary thyroid carcinoma.
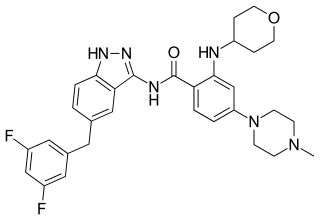
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).
Lipofibromatosis-like neural tumor (LPF-NT) is an extremely rare soft tissue tumor first described by Agaram et al in 2016. As of mid-2021, at least 39 cases of LPF-NT have been reported in the literature. LPF-NT tumors have several features that resemble lipofibromatosis (LPF) tumors, malignant peripheral nerve sheath tumors, spindle cell sarcomas, low-grade neural tumors, peripheral nerve sheath tumors, and other less clearly defined tumors; Prior to the Agaram at al report, LPF-NTs were likely diagnosed as variants or atypical forms of these tumors. The analyses of Agaram at al and subsequent studies uncovered critical differences between LPF-NT and the other tumor forms which suggest that it is a distinct tumor entity differing not only from lipofibromatosis but also the other tumor forms.
ACD856, or ACD-856, is a tropomyosin receptor kinase TrkA, TrkB, and TrkC positive allosteric modulator which is under development for the treatment of Alzheimer's disease, depressive disorders, sleep disorders, and traumatic brain injuries. It is taken by mouth.