
Etonitazene, also known as EA-4941 or CS-4640, is a benzimidazole opioid, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.

Rolicyclidine (PCPy) is a dissociative anesthetic that is similar in effects to phencyclidine, but is slightly less potent and has fewer stimulant effects. It instead produces a sedative effect described as being somewhat similar to a barbiturate, but with additional PCP-like dissociative, anaesthetic and hallucinogenic effects. Due to its similarity in effects to PCP, PCPy was placed into the Schedule I list of illegal drugs in the 1970s, although it has never been widely abused and is now little known.

Eticyclidine is a dissociative anesthetic drug with hallucinogenic effects. It is similar in effects to phencyclidine but is slightly more potent. PCE was developed by Parke-Davis in the 1970s and evaluated for anesthetic potential under the code name CI-400, but research into PCE was not continued after the development of ketamine, a similar drug with more favourable properties. Due to its similarity in effects to PCP, PCE was placed into the Schedule 1 list of illegal drugs in the 1970s, although it was only briefly abused in the 1970s and 1980s and is now little known.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Azaprocin is a drug which is an opioid analgesic with approximately ten times the potency of morphine, and a fast onset and short duration of action. It was discovered in 1963, but has never been marketed.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

BDPC is a potent fully synthetic opioid with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the potency of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active trans-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug market internationally. Analogues where the para-bromine is replaced by chlorine or a methyl group retain similar activity, while the meta-hydroxyl derivative demonstrated robust antagonist activity.

(–)-2β-Carbomethoxy-3β-(4-tolyl)tropane is a phenyltropane-based cocaine analogue that has similar properties in vitro to related drugs such as RTI-31.
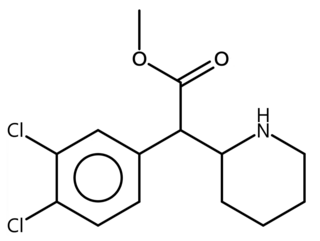
3,4-dichloromethylphenidate is a potent stimulant drug from the phenidate class closely related to methylphenidate. It acts as a potent serotonin-norepinephrine-dopamine reuptake inhibitor with a long duration of action. It has been sold online as a designer drug.
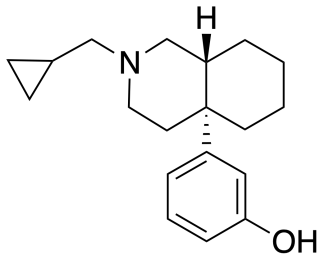
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol, with a number of other related compounds known. Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.

U-47700, also known as U4, pink heroin, pinky, and pink, is an opioid analgesic drug developed by a team at Upjohn in the 1970s which has around 7.5 times the potency of morphine in animal models.

The ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues into the cell. This technology was invented by Professor Chris McGuigan from the School of Pharmacy and Pharmaceutical Sciences at Cardiff University in the early 1990s. ProTides form a critical part of antiviral drugs such as sofosbuvir, tenofovir alafenamide, and remdesivir.
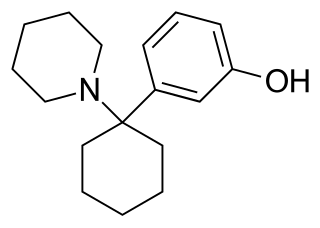
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug.
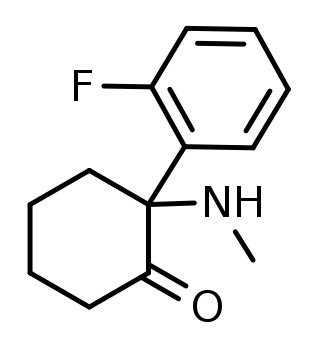
2-Fluorodeschloroketamine is a dissociative anesthetic related to ketamine. Its sale and use as a designer drug has been reported in various countries. It is an analogue of ketamine where the chlorine group has been replaced by fluorine. Due to its recent emergence, the pharmacological specifics of the compound are mostly unclear, but effects are reported to be similar to its parent compound, ketamine.

3-Methyl-PCP is a recreational designer drug with dissociative effects. It is an arylcyclohexylamine derivative, related to drugs such as 3'-MeO-PCP and 3'-Me-PCPy. It was first synthesised in the 1960s, but was only identified on the illicit market in Hungary in September 2020, and was made illegal in Hungary in April 2021.

Fentanyl tropane (tropafentanyl) is an opioid derivative which is an analogue of fentanyl, where the central piperidine ring has been bridged with a two carbon chain to form a tropane ring. It is made from tropinone in a similar manner to how fentanyl is made from 4-piperidone, and has two isomers, with the 3β isomer being around half the potency of fentanyl while the 3α isomer is much weaker, only around the same potency as morphine.

U-32,802A is an antipsychotic tranquilizer of the butyrophenone class. It was developed by Daniel Lednicer at Upjohn Pharmaceuticals in the early part of the 1970’s. Whereas related agents are based on a 4-aryl-piperidine central ring system, U-32,802A is an 4-aryl-cyclohexylamine, which might be expected to offer a unique pharmacological profile. Based on the available pharmacological data, U-32,802A was hypothesized to act primarily at the presynaptic organelles for storage of monoamines in a way similar to tetrabenazine.

















