
Pergolide, sold under the brand name Permax and Prascend (veterinary) among others, is an ergoline-based dopamine receptor agonist used in some countries for the treatment of Parkinson's disease. Parkinson's disease is associated with reduced dopamine synthesis in the substantia nigra of the brain. Pergolide acts on many of the same receptors as dopamine to increase receptor activity.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Noradrenergic and specific serotonergic antidepressants (NaSSAs) are a class of psychiatric drugs used primarily as antidepressants. They act by antagonizing the α2-adrenergic receptor and certain serotonin receptors such as 5-HT2A and 5-HT2C, but also 5-HT3, 5-HT6, and/or 5-HT7 in some cases. By blocking α2-adrenergic autoreceptors and heteroreceptors, NaSSAs enhance adrenergic and serotonergic neurotransmission in the brain involved in mood regulation, notably 5-HT1A-mediated transmission. In addition, due to their blockade of certain serotonin receptors, serotonergic neurotransmission is not facilitated in unwanted areas, which prevents the incidence of many side effects often associated with selective serotonin reuptake inhibitor (SSRI) antidepressants; hence, in part, the "specific serotonergic" label of NaSSAs.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.

EX-597 is a fatty acid amide hydrolase inhibitor which is under development for the treatment of social anxiety disorder and post-traumatic stress disorder (PTSD).

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

5-hydroxytryptamine receptor 1B also known as the 5-HT1B receptor is a protein that in humans is encoded by the HTR1B gene. The 5-HT1B receptor is a 5-HT receptor subtype.
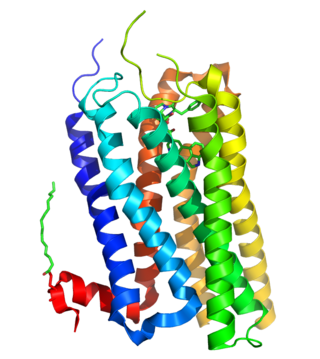
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

Flesinoxan (DU-29,373) is a potent and selective 5-HT1A receptor partial/near-full agonist of the phenylpiperazine class. Originally developed as a potential antihypertensive drug, flesinoxan was later found to possess antidepressant and anxiolytic effects in animal tests. As a result, it was investigated in several small human pilot studies for the treatment of major depressive disorder, and was found to have robust effectiveness and very good tolerability. However, due to "management decisions", the development of flesinoxan was stopped and it was not pursued any further.
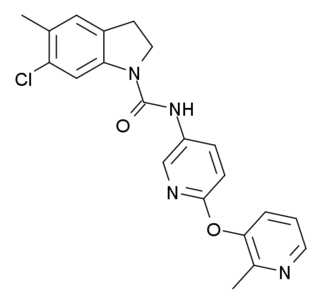
SB-242084 is a psychoactive drug and research chemical which acts as a selective antagonist for the 5HT2C receptor. It has anxiolytic effects, and enhances dopamine signalling in the limbic system, as well as having complex effects on the dopamine release produced by cocaine, increasing it in some brain regions but reducing it in others. It has been shown to increase the effectiveness of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants, and may also reduce their side effects. In animal studies, SB-242084 produced stimulant-type activity and reinforcing effects, somewhat similar to but much weaker than cocaine or amphetamines.

Lecozotan is an investigational drug by Wyeth tested for improvement of cognitive functions of Alzheimer's disease patients. As of June 2008, the first Phase III clinical trial has been completed.

AR-A000002, also known as AZD-8129, is a drug which is one of the first compounds developed to act as a selective antagonist for the serotonin receptor 5-HT1B, with approximately 10x selectivity for 5-HT1B over the closely related 5-HT1D receptor. It has been shown to produce sustained increases in levels of serotonin in the brain, and has anxiolytic effects in animal studies.

RS-102221 is a drug developed by Hoffmann–La Roche, which was one of the first compounds discovered that acts as a potent and selective antagonist at the serotonin 5-HT2C receptor, with around 100× selectivity over the closely related 5-HT2A and 5-HT2B receptors. It has anxiolytic effects in animal studies, increases the effectiveness of SSRI antidepressants, and shows a complex interaction with cocaine, increasing some effects but decreasing others, reflecting a role for the 5-HT2C receptor in regulation of the dopamine signalling system in the brain.

SB-216641 is a drug which is a selective antagonist for the serotonin receptor 5-HT1B, with around 25x selectivity over the closely related 5-HT1D receptor. It is used in scientific research, and has demonstrated anxiolytic effects in animal studies.
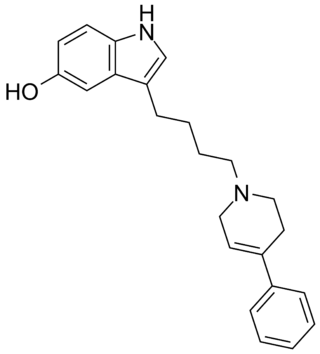
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.

CP-93129 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 150x and 200x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It is used in the study of 5-HT1B receptors in the brain, particularly their role in modulating the release of other neurotransmitters.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.

S32212 is a drug which is under preclinical investigation as a potential antidepressant medicine. It behaves as a selective, combined 5-HT2C receptor inverse agonist and α2-adrenergic receptor antagonist (at all three subtypes—α2A, α2B, and α2C) with additional 5-HT2A and, to a lesser extent, 5-HT2B receptor antagonistic properties, and lacks any apparent affinity for the monoamine reuptake transporters or for the α1-adrenergic, H1, or mACh receptors. This profile of activity is compatible with the definition of a noradrenergic and specific serotonergic antidepressant (NaSSA), and as such, S32212 could in turn be classified as a NaSSA.

WAY-163,909 is a drug which acts as a potent and reasonably selective agonist for the serotonin 5-HT2C receptor. It has antipsychotic-like effects in animal models, and has been used to study the role of the 5-HT2C receptor subtype in the action of addictive drugs such as nicotine and methamphetamine.

NAS-181, also known as MCOMM, is a selective rodent serotonin 5-HT1B receptor antagonist which is used in scientific research.


















