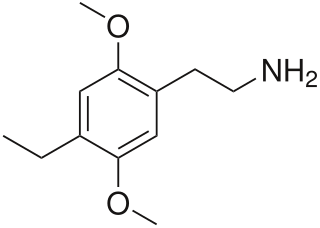
2C-E is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and documented in his book PiHKAL. Like the other substances in its family, it produces sensory and cognitive effects in its physical reactions with living organisms.

2C-T-2 is a psychedelic and entactogenic phenethylamine of the 2C family. It was first synthesized in 1981 by Alexander Shulgin, and rated by him as one of the "magical half-dozen" most important psychedelic phenethylamine compounds. The drug has structural and pharmacodynamic properties similar to those of 2C-T-7.

2C-TFM is a psychedelic phenethylamine of the 2C family. It was first synthesized in the laboratory of David E. Nichols. It has also been called 2C-CF3, a name derived from the Para-trifluoromethyl group it contains.
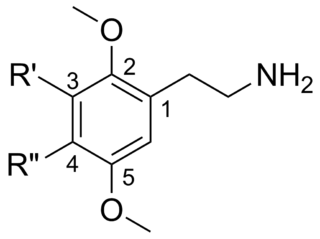
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring. Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds. Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL. Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.

2C-T-15 or 2,5-dimethoxy-4-(β-cyclopropylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .
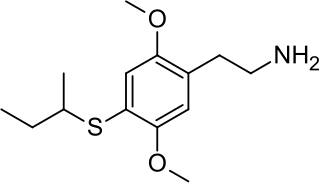
2C-T-17 or 2,5-dimethoxy-4-(β-secbutylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .

2C-H (2,5-dimethoxyphenethylamine) is a lesser-known substituted phenethylamine of the 2C family.

Ariadne is a little-known psychoactive drug. It is a homologue of the psychedelics 2C-D and DOM. Ariadne was first synthesized by Alexander Shulgin. In his 1991 book PiHKAL, Shulgin reported testing Ariadne on himself up to a dose of 32 mg, finding that it produced "the alert of a psychedelic, with none of the rest of the package". Very little published data exists about the human pharmacology of Ariadne apart from Shulgin's limited testing; unpublished human trials reportedly observed some psychoactive effects, but no hallucinations.

2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine is a lesser-known psychedelic drug and member of the DOx class. DOEF was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 2–3.5 mg, and the duration is listed as 12–16 hours. Very little data exists about the pharmacological properties, metabolism, and toxicity of DOEF.

2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. Most compounds of this class are potent and long-lasting psychedelic drugs, and act as highly selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonists. A few bulkier derivatives such as DOAM have similarly high binding affinity for 5-HT2 receptors but instead act as antagonists, and so do not produce psychedelic effects though they retain amphetamine-like stimulant effects.
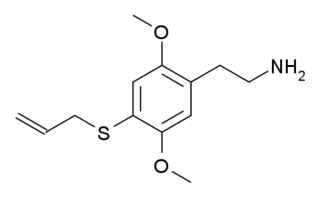
2C-T-16 is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL, however while Shulgin began synthesis of this compound he only got as far as the nitrostyrene intermediate, and did not complete the final synthetic step. Synthesis of 2C-T-16 was finally achieved by Daniel Trachsel some years later, and it was subsequently reported as showing similar psychedelic activity to related compounds, with a dose range of 10–25 mg and a duration of 4–6 hours, making it around the same potency as the better-known saturated analogue 2C-T-7, but with a significantly shorter duration of action. Binding studies in vitro showed 2C-T-16 to have a binding affinity of 44 nM at 5-HT2A and 15 nM at 5-HT2C. 2C-T-16 and related derivatives are potent partial agonists of the 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors and induce a head-twitch response in mice.

2,5-Dimethoxy-4-isopropylamphetamine is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL. Shulgin described DOiPR as being at least an order of magnitude weaker than DOPr, with doses of 20–30 mg required to produce valid changes in mental state. Very little data exists about the pharmacological properties, metabolism, and toxicity of DOiPR.

2C-Se is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL. Shulgin considered 2C-Se to be around three times the potency of mescaline, but was too concerned about toxicity to test it extensively, though he considered it noteworthy as the only psychedelic drug to contain a selenium atom.

2C-EF is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL, but he never synthesised it himself, though it has been synthesised and its activity tested in subsequent years.

4C-B is a lesser-known psychedelic drug which is related to 2C-B and DOB. It is a reasonably potent 5-HT2A receptor partial agonist with a Ki of 7.6nM, but has relatively low efficacy. It is briefly mentioned in Alexander Shulgin's book PiHKAL but was never tested by him, however it has subsequently been tested by other researchers and was found to be active in a dose range of 50-80mg with a duration of around 8 hours, though with generally milder effects than 2C-B or DOB.

2C-T-3 is a lesser-known psychedelic drug related to compounds such as 2C-T-7 and 2C-T-16. It was named by Alexander Shulgin but was never made or tested by him, and was instead first synthesised by Daniel Trachsel some years later. It has a binding affinity of 11nM at 5-HT2A and 40nM at 5-HT2C. It is reportedly a potent psychedelic drug with an active dose in the 15–40 mg range, and a duration of action of 8–14 hours, with visual effects comparable to related drugs such as methallylescaline.

2C-T-28 is a lesser-known psychedelic drug related to compounds such as 2C-T-7 and 2C-T-21. It was named by Alexander Shulgin but was never made or tested by him, and was instead first synthesised by Daniel Trachsel some years later. It has a binding affinity of 75 nM at 5-HT2A and 28 nM at 5-HT2C. It is reportedly a potent psychedelic drug with an active dose in the 8–20 mg range, and a duration of action of 8–10 hours, with prominent visual effects. 2C-T-28 is the 3-fluoropropyl instead of 2-fluoroethyl chain-lengthened homologue of 2C-T-21 and has very similar properties, although unlike 2C-T-21 it will not form toxic fluoroacetate as a metabolite.

CYB210010 (2C-T-TFM) is a lesser-known psychedelic drug related to compounds such as 2C-T and 2C-T-21. Alexander Shulgin attempted to synthesise this compound in the 1990s, and mentions it in his book PiHKAL under the entry for 2C-T-21, but was unsuccessful in producing a key intermediate and never assigned it a 2C-T number. This compound was ultimately first synthesised by Geoffrey Varty and colleagues at Irish biopharmaceutical company Cybin in 2023. It has a Ki of 0.35 nM at 5-HT2A, and an EC50 of 4.1 nM at 5-HT2A and 7.3 nM at 5-HT2C, compared to 88 nM at 5-HT2B. It is a potent, selective, long acting and orally active agonist for the 5-HT2A and 5-HT2C receptors and produces psychedelic-like responding in several different animal species. It is not known to have been tested in humans.




















