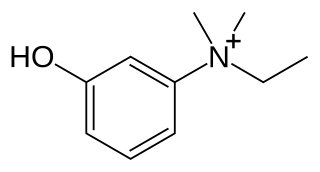
Edrophonium is a readily reversible acetylcholinesterase inhibitor. It prevents breakdown of the neurotransmitter acetylcholine and acts by competitively inhibiting the enzyme acetylcholinesterase, mainly at the neuromuscular junction. It is sold under the trade names Tensilon and Enlon.

Oxandrolone, sold under the brand names Oxandrin and Anavar, among others, is an androgen and anabolic steroid (AAS) medication which is used to help promote weight gain in various situations, to help offset protein catabolism caused by long-term corticosteroid therapy, to support recovery from severe burns, to treat bone pain associated with osteoporosis, to aid in the development of girls with Turner syndrome, and for other indications. It is taken by mouth.

Anna Hoxha, known professionally as Anna Oxa, is an Italian singer, actress and television presenter. She has received mainstream popularity and recognition within Italy due to her numerous participations in the Sanremo Music Festival.
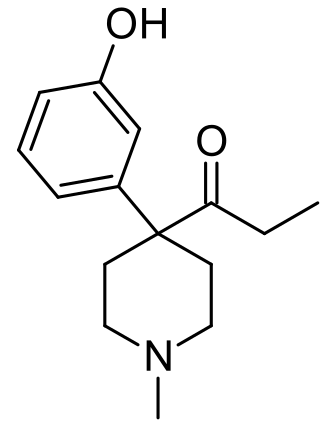
Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Iceland, Norway and Sweden and is used for severe pain.

HPTE, also known as hydroxychlor, p,p'-hydroxy-DDT, or 2,2-bis(4-hydroxyphenyl)-1,1,1-trichloroethane, is a metabolite of methoxychlor, a synthetic insecticide related to DDT. Like bisphenol A with similar chemical structure, HPTE is an endocrine disruptor which has estrogenic activity, and also inhibits Cholesterol side-chain cleavage enzyme and 3α-hydroxysteroid dehydrogenase (3α-HSD).

Latamoxef is an oxacephem antibiotic usually grouped with the cephalosporins. In oxacephems such as latamoxef, the sulfur atom of the cephalosporin core is replaced with an oxygen atom.
The bisphenols are a group of chemical compounds related to diphenylmethane. Most are based on two hydroxyphenyl functional groups linked by a methylene bridge. Exceptions include bisphenol S, P, and M. "Bisphenol" is a common name; the letter following denotes the variant, which depends on the additional substituents. Bisphenol A is the most popular representative of the group, often simply called "bisphenol".

Atiprimod is a substance being studied in the treatment of certain multiple myelomas and other advanced cancers. It may block the growth of tumors and the growth of blood vessels from surrounding tissue to the tumor. This drug is also being researched as a potential treatment for various autoimmune diseases. It was first developed by GlaxoSmithKline as a potential treatment for rheumatoid arthritis. The substance is also known as azaspirane, although this more properly refers to the class of chemicals to which atiprimod belongs.

Gadobenic acid is a complex of gadolinium with the ligand BOPTA. In the form of the methylglucamine salt meglumine gadobenate (INNm) or gadobenate dimeglumine (USAN), it is used as a gadolinium-based MRI contrast medium.

Nidula is a genus of fungi in the family Agaricaceae. Their fruit bodies resemble tiny egg-filled birds' nests, from which they derive their common name "bird's nest fungi". Originally described in 1902, the genus differs from the related genera Cyathus and Crucibulum by the absence of a cord that attaches the eggs to the inside of the fruit body. The life cycle of this genus allows it to reproduce both sexually, with meiosis, and asexually via spores. Species in this genus produce a number of bioactive compounds, including 4-(p-hydroxyphenyl)-2-butanone, a major component of raspberry flavor and insect attractor used in pesticides.
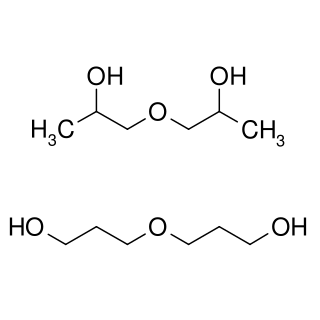
Dipropylene glycol is a mixture of three isomeric chemical compounds, 4-oxa-2,6-heptandiol, 2-(2-hydroxy-propoxy)-propan-1-ol, and 2-(2-hydroxy-1-methyl-ethoxy)-propan-1-ol. It is a colorless, nearly odorless liquid with a high boiling point and low toxicity.

Fenadiazole (INN), also known as phénadiazole (DCF) and sold under the brand names Hypnazol, Eudormil, and Viodor, is a hypnotic and sedative medication which has been used to treat insomnia but is no longer marketed. It is described as a non-barbiturate hypnotic with marked or profound hypnotic and sedative properties in animals, variable hypnotic effects in humans, additional anticonvulsant, antithermal, and spasmolytic effects, and a generally well-tolerated profile in humans. The drug was synthesized, pharmacologically characterized, patented, and marketed by the French pharmaceutical company Laboratoires Jacques Logeais between 1960 and 1962. As a hypnotic and sedative, fenadiazole has a unique oxadiazole-based chemical structure. It may be chemically related to certain other hypnotics and sedatives with atypical chemical structures.

LY-379,268 is a drug that is used in neuroscience research, which acts as a potent and selective agonist for the group II metabotropic glutamate receptors (mGluR2/3).

An oxaprostaglandin is a type of prostaglandin with one carbon atom replaced by an oxygen atom. These are found in nature and have also been produced synthetically.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs. It is given by mouth.
1,7-Bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one is a natural product, a curcuminoid antioxidant found in turmeric and torch ginger.
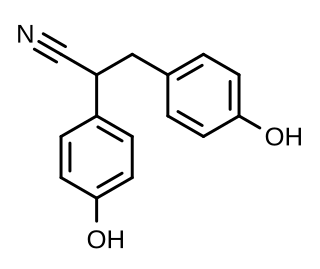
Diarylpropionitrile (DPN), also known as 2,3-bis(p-hydroxyphenyl)propionitrile (2,3-BHPPN), is a synthetic, nonsteroidal, and highly selective agonist of ERβ (IC50 = 15 nM) that is used widely in scientific research to study the function of this receptor. It is 70-fold more selective for ERβ over ERα, and has 100-fold lower affinity for GPER (GPR30) relative to estradiol. DPN produces antidepressant- and anxiolytic-like effects in animals via activation of the endogenous oxytocin system. First reported in 2001, DPN was the first selective ERβ agonist to be discovered, and was followed by prinaberel (ERB-041, WAY-202041), WAY-200070, and 8β-VE2 in 2004, ERB-196 (WAY-202196) in 2005, and certain phytoestrogens like liquiritigenin and nyasol (cis-hinokiresinol) since 2007.

Osaterone, also known as 17α-hydroxy-6-chloro-2-oxa-6-dehydroprogesterone, as well as 2-oxachloromadinone, is a steroidal antiandrogen and progestin that was never marketed. The C17α acetate ester of osaterone, osaterone acetate, in contrast, has been marketed.

BOMT, also known by its developmental code name Ro 7-2340 and as 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone, is a synthetic steroidal antiandrogen which was first developed in 1970 and was never marketed for medical use. It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of the androgen dihydrotestosterone (DHT). Along with benorterone, cyproterone, and flutamide, BOMT was among the earliest antiandrogens to be developed and extensively studied, although it is less well-documented in comparison to the others. BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue. There was also interest in BOMT for the potential applications of acne, pattern hair loss, and possibly prostate cancer, but it was not developed for these indications either.

3-PPP (N-n-propyl-3-(3-hydroxyphenyl)piperidine) is a mixed sigma σ1 and σ2 receptor agonist (with similar affinity for both subtypes, though slightly higher affinity for the latter) and D2 receptor partial agonist which is used in scientific research. It shows stereoselectivity in its pharmacodynamics. (+)-3-PPP is the enantiomer that acts as an agonist of the sigma receptors; it is also an agonist of both D2 presynaptic and postsynaptic receptors. Conversely, (–)-3-PPP, also known as preclamol (INN), acts as an agonist of presynaptic D2 receptors but as an antagonist of postsynaptic D2 receptors, and has antipsychotic effects. 3-PPP has also been reported to be a monoamine reuptake inhibitor and possibly to act at adrenergic receptors or some other non-sigma receptor.



















