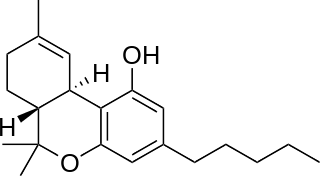
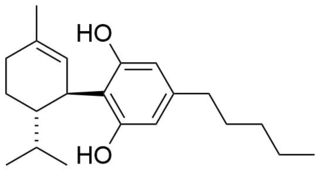
Tetrahydrocannabinol (THC) is a cannabinoid found in cannabis. It is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. It is a colorless oil.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 100 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.
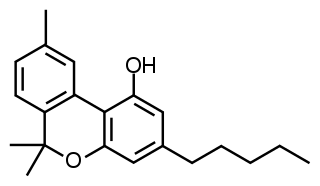
Cannabinol (CBN) is a mildly psychoactive phytocannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.

Parahexyl, also known as synhexyl, is a synthetic homologue of tetrahydrocannabinol (THC) which was invented in 1941 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis.
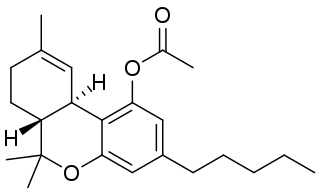
THC-O-acetate is the acetate ester of THC. The term THC-O-acetate and its variations are commonly used for two types of the substance, dependent on which cannabinoid it is synthesized from. The difference between Δ8-THC and Δ9-THC is bond placement on the cyclohexene ring.

11-Hydroxy-Δ9-tetrahydrocannabinol, usually referred to as 11-hydroxy-THC is the main active metabolite of tetrahydrocannabinol (THC), which is formed in the body after Δ9-THC is consumed.

Cannabigerol (CBG) is a non-psychoactive cannabinoid and minor constituent of cannabis. It is one of more than 120 identified cannabinoids found in the plant genus Cannabis. The compound is the decarboxylated form of cannabigerolic acid (CBGA), the parent molecule from which other cannabinoids are biosynthesized.
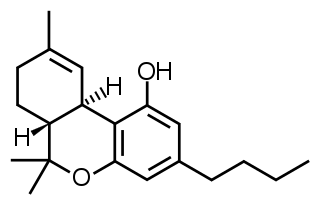
Δ9-Tetrahydrocannabutol is a phytocannabinoid found in cannabis that is a homologue of tetrahydrocannabinol (THC), the main active component of Cannabis. Structurally, they are only different by the pentyl side chain being replaced by a butyl side chain. THCB was studied by Roger Adams as early as 1942

Dronabinol, sold under the brand names Marinol and Syndros, is the generic name for the molecule of (−)-trans-Δ9-tetrahydrocannabinol (THC) in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the U.S. Food and Drug Administration (FDA) as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting.

Tetrahydrocannabinolic acid is a precursor of tetrahydrocannabinol (THC), an active component of cannabis.
8,9-Dihydrocannabidiol is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. that was first synthesized by Alexander R. Todd in 1940 derived from the catalytic hydrogenation of cannabidiol.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 receptor agonist which was known as a synthetic homologue of tetrahydrocannabinol (THC), but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa.

Δ-3-Tetrahydrocannabinol is a synthetic isomer of tetrahydrocannabinol (THC) developed during the original research in the 1940s to develop synthetic routes to the natural products Δ8-THC and Δ9-THC found in the cannabis. While the normal trans configuration of THC is in this case flattened by the double bond, it still has two enantiomers as the 9-methyl group can exist in an (R) or (S) conformation. The (S) enantiomer has similar effects to Δ9-THC though with several times lower potency, while the (R) enantiomer is many times less active or inactive, depending on the assay used. It has been identified as a component of vaping liquid products.

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by firstly acid cyclization of cannabidiol and then hydrogenation of tetrahydrocannabinol. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams.

11-Hydroxy-Δ-8-tetrahydrocannabinol is an active metabolite of Δ8-THC, a psychoactive cannabinoid found in small amounts in cannabis. It is an isomer of 11-OH-Δ9-THC, and is produced via the same metabolic pathway. It was the first cannabinoid metabolite discovered in 1970.

Tetrahydrocannabihexol is a phytocannabinoid, the hexyl homologue of tetrahydrocannabinol (THC) which was first isolated from Cannabis plant material in 2020 along with the corresponding hexyl homologue of cannabidiol, though it had been known for several decades prior to this as an isomer of the synthetic cannabinoid parahexyl. Another isomer Δ8-THCH is also known as a synthetic cannabinoid under the code number JWH-124, though it is unclear whether this occurs naturally in Cannabis, but likely is due to Δ8-THC itself being a degraded form of Δ9-THC. THC-Hexyl can be synthesized from 4-hexylresorcinol and was studied by Roger Adams as early as 1942.
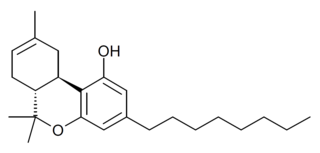
JWH-138 (THC-Octyl, Δ8-THC-C8) is a synthetic cannabinoid first synthesized by Roger Adams and studied heavily by John W. Huffman, with a Ki of 8.5nM at the CB1 cannabinoid receptor. THC-Octyl and its hydrogenated analog HHC-Octyl was synthesized and studied by Roger Adams as early as 1942.
Conversion of cannabidiol (CBD) to tetrahydrocannabinol (THC) can occur through a ring-closing reaction. This cyclization can be acid-catalyzed or brought about by heating.
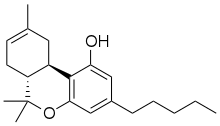
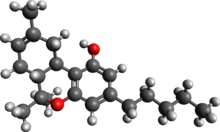
![[?] -THC has a double bond (a) between the carbon atoms labeled 8 and 9. Delta-8-THC.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Delta-8-THC.jpg/344px-Delta-8-THC.jpg)
![[?] -THC has a double bond (a) between the carbon atoms labeled 9 and 10. Delta-9-THC.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e1/Delta-9-THC.jpg/344px-Delta-9-THC.jpg)

















