PiHKAL: A Chemical Love Story is a book by Dr. Alexander Shulgin and Ann Shulgin, published in 1991. The subject of the work is psychoactive phenethylamine chemical derivatives, notably those that act as psychedelics and/or empathogen-entactogens. The main title, PiHKAL, is an acronym that stands for "Phenethylamines I Have Known and Loved".
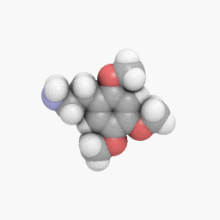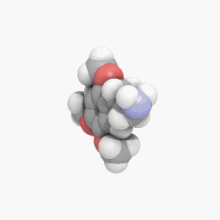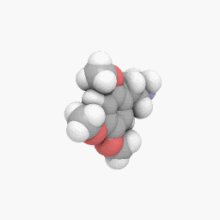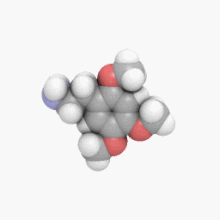
2C-E is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and documented in his book PiHKAL. Like the other substances in its family, it produces sensory and cognitive effects in its physical reactions with living organisms.

2C-N (2,5-dimethoxy-4-nitrophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin.

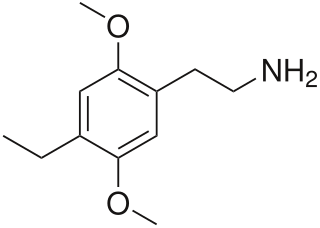
Escaline (3,5-methoxy-4-ethoxyphenethylamine) is a psychedelic drug and entheogen of the phenethylamine class of compounds. Escaline was first synthesized and reported in the scientific literature by Benington, et al., in 1954, but was later re-examined in the laboratory of David E. Nichols, who prepared a series of mescaline analogues that included escaline, proscaline, and isoproscaline. The effects of this and related mescaline analogues in humans were first described by Alexander Shulgin. In his book PiHKAL , Shulgin lists the dosage range as 40 to 60 mg, consumed orally. The duration of action was stated to be 8–12 hours.

2C-T-4 (2,5-dimethoxy-4-isopropylthiophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and is used as entheogenic recreational drug.

2C-T-19 (2,5-dimethoxy-4-butylthiophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin.
Trimethoxyamphetamines (TMAs) are a family of isomeric psychedelic hallucinogenic drugs. There exist six different TMAs that differ only in the position of the three methoxy groups: TMA, TMA-2, TMA-3, TMA-4, TMA-5, and TMA-6. The TMAs are analogs of the phenethylamine cactus alkaloid mescaline. The TMAs are substituted amphetamines, however, their mechanism of action is more complex than that of the unsubstituted compound amphetamine, probably involving agonist activity on serotonin receptors such as the 5HT2A receptor in addition to the generalised dopamine receptor agonism typical of most amphetamines. This action on serotonergic receptors likely underlie the psychedelic effects of these compounds. It is reported that some TMAs elicit a range of emotions ranging from sadness to empathy and euphoria. TMA was first synthesized by Hey, in 1947. Synthesis data as well as human activity data has been published in the book PiHKAL.

2C-G is a psychedelic phenethylamine of the 2C family. First synthesized by Alexander Shulgin, it is sometimes used as an entheogen. It has structural and pharmacodynamic properties similar to 2C-D and Ganesha. Like many of the phenethylamines in PiHKAL, 2C-G and its homologs have only been taken by Shulgin and a small test group, making it difficult to ensure completeness when describing effects.
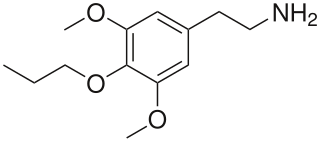
Proscaline (4-propoxy-3,5-DMPEA) is a psychedelic and hallucinogenic drug. It has structural properties similar to the drugs mescaline, isoproscaline, and escaline. In PiHKAL, Alexander Shulgin reports that a dose of 30–60 mg produces effects lasting 8–12 hours.

2C-T is a psychedelic and hallucinogenic drug of the 2C family. It is used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs mescaline and 2C-T-2.
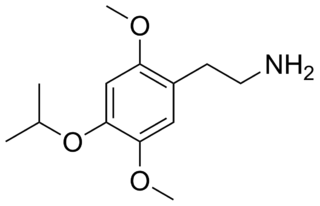
2C-O-4 (4-isopropoxy-2,5-dimethoxyphenethylamine) is a phenethylamine of the 2C family. It is also a positional isomer of isoproscaline and was probably first synthesized by Alexander Shulgin. It produces hallucinogenic, psychedelic, and entheogenic effects. Because of the low potency of 2C-O-4, and the inactivity of 2C-O, Shulgin felt that the 2C-O series would not be an exciting area for research, and did not pursue any further analogues.

2C-T-13 is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL.

2C-T-15 or 2,5-dimethoxy-4-(β-cyclopropylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .
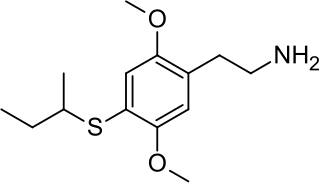
2C-T-17 or 2,5-dimethoxy-4-(β-secbutylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .

2C-H (2,5-dimethoxyphenethylamine) is a lesser-known substituted phenethylamine of the 2C family.

BOD (4-methyl-2,5,β-trimethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy analog of 2C-D. BOD was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 15–25 mg, and the duration listed as 8–16 hours. BOD produces strongly distorted open-eye visuals, and some closed-eye visuals. It also has an entheogenic effect and produces humor. Very little data exists about the pharmacological properties, metabolism, and toxicity of BOD.

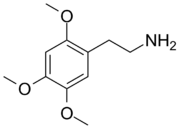
3,4-Dimethoxyphenethylamine (DMPEA) is a chemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine.
Dimethoxyamphetamine (DMA) is a series of six lesser-known psychedelic drugs similar in structure to the three isomers of methoxyamphetamine and six isomers of trimethoxyamphetamine. The isomers are 2,3-DMA, 2,4-DMA, 2,5-DMA, 2,6-DMA, 3,4-DMA, and 3,5-DMA. Three of the isomers were characterized by Alexander Shulgin in his book PiHKAL. Little is known about their dangers or toxicity.
Trimethoxyphenethylamines (TMPEA) are a group of positional isomers of the psychedelic cactus alkaloid mescaline. Some of them are described in the book PiHKAL by Alexander Shulgin and Ann Shulgin.
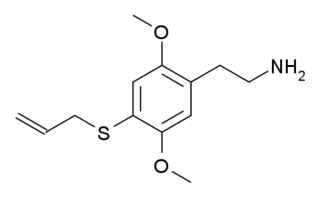
2C-T-16 is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL, however while Shulgin began synthesis of this compound he only got as far as the nitrostyrene intermediate, and did not complete the final synthetic step. Synthesis of 2C-T-16 was finally achieved by Daniel Trachsel some years later, and it was subsequently reported as showing similar psychedelic activity to related compounds, with a dose range of 10–25 mg and a duration of 4–6 hours, making it around the same potency as the better-known saturated analogue 2C-T-7, but with a significantly shorter duration of action. Binding studies in vitro showed 2C-T-16 to have a binding affinity of 44nM at 5-HT2A and 15nM at 5-HT2C. 2C-T-16 and related derivatives are potent partial agonists of the 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors and induce a head-twitch response in mice.