
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties react to form a β-hydroxyaldehyde or β-hydroxyketone, and this is then followed by dehydration to give a conjugated enone.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words lactone + amide.
Calcium hypochlorite is an inorganic compound with chemical formula Ca(ClO)2, also written as Ca(OCl)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. It is named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978). The method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
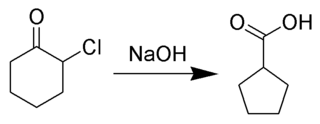
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds. Note that trihalomethyl ketone substrates will result in haloform and carboxylate formation via the haloform reaction instead.
The Weinreb ketone synthesis or Weinreb–Nahm ketone synthesis is a chemical reaction used in organic chemistry to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones. The original reaction involved two subsequent substitutions: the conversion of an acid chloride with N,O-Dimethylhydroxylamine, to form a Weinreb–Nahm amide, and subsequent treatment of this species with an organometallic reagent such as a Grignard reagent or organolithium reagent. Nahm and Weinreb also reported the synthesis of aldehydes by reduction of the amide with an excess of lithium aluminum hydride.

The Finkelstein reaction, named after the German chemist Hans Finkelstein, is a type of SN2 reaction that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the reaction can be driven to completion by exploiting the differential solubility of various halide salts, or by using a large excess of the desired halide.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.

In chemistry, the haloform reaction is a chemical reaction in which a haloform is produced by the exhaustive halogenation of an acetyl group, in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform, bromoform, or iodoform. Note that fluoroform can't be prepared in this way.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.

Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis.
In organic chemistry, the Corey–Link reaction is a name reaction that converts a 1,1,1-trichloro-2-keto structure into a 2-aminocarboxylic acid or other acyl functional group with control of the chirality at the alpha position. The reaction is named for E.J. Corey and John Link, who first reported the reaction sequence.
In organic chemistry, the Jocic reaction, also called the Jocic–Reeve reaction is a name reaction that generates α-substituted carboxylic acids from trichloromethylcarbinols and corresponding nucleophiles in the presence of sodium hydroxide. The reaction involves nucleophilic displacement of the hydroxyl group in a 1,1,1-trichloro-2-hydroxyalkyl structure with concomitant conversion of the trichloromethyl portion to a carboxylic acid or other acyl group.

















