In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (NH3), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as monochloramine (NClH2).

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers.

Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character.
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.
In chemistry, the phosphonium cation describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
The Wurtz reaction, named after Charles Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main-group polymers, whereby two alkyl halides are reacted with sodium metal in dry ether solution to form a higher alkane. In this reaction alkyl halides are treated with sodium metal in dry ethereal (free from moisture) solution to produce higher alkanes. It is also used for the preparation of higher alkanes containing even number of carbon atoms:
The Gabriel synthesis is a chemical reaction that transforms primary alkyl halides into primary amines. Traditionally, the reaction uses potassium phthalimide. The reaction is named after the German chemist Siegmund Gabriel.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed, usually as a base, in organic synthesis.
A 1,2-Wittig rearrangement is a categorization of chemical reactions in organic chemistry, and consists of a 1,2-rearrangement of an ether with an alkyllithium compound. The reaction is named for Nobel Prize winning chemist Georg Wittig.

The Michaelis–Arbuzov reaction is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the Arbuzov reaction; phosphite esters (1) react to form phosphonates (2), phosphonites (3) react to form phosphinates (4) and phosphinites (5) react to form phosphine oxides (6).
In organic chemistry, the Menshutkin reaction converts a tertiary amine into a quaternary ammonium salt by reaction with an alkyl halide. Similar reactions occur when tertiary phosphines are treated with alkyl halides.
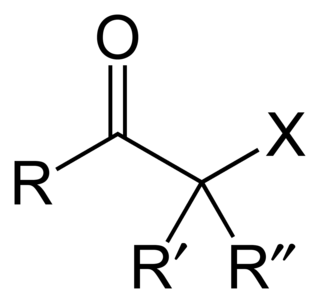
In organic chemistry, an α-haloketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-haloketones are alkylating agents. Prominent α-haloketones include phenacyl bromide and chloroacetone.
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.

The Delépine reaction is the organic synthesis of primary amines (4) by reaction of benzyl or alkyl halides (1) with hexamethylenetetramine (2) followed by acid hydrolysis of the quaternary ammonium salt (3). It is named after the French chemist Stéphane Marcel Delépine (1871–1965).

The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor or another electrophilic alkylation reagent to give an alkylated iminium product, which is hydrolyzed by dilute aqueous acid to give the alkylated ketone or aldehyde. Since enamines are generally produced from ketones or aldehydes, this overall process constitutes a selective monoalkylation of a ketone or aldehyde, a process that may be difficult to achieve directly.

Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry.
The Willgerodt rearrangement or Willgerodt reaction is an organic reaction converting an aryl alkyl ketone, alkyne, or alkene to the corresponding amide by reaction with ammonium polysulfide, named after Conrad Willgerodt. The formation of the corresponding carboxylic acid is a side reaction. When the alkyl group is an aliphatic chain, multiple reactions take place with the amide group always ending up at the terminal end.
The Kolbe nitrile synthesis is a method for the preparation of alkyl nitriles by reaction of the corresponding alkyl halide with a metal cyanide. A side product for this reaction is the formation of an isonitrile because the cyanide ion is an ambident nucleophile and according to Kornblum's rule is capable of reacting with either carbon or nitrogen. The reaction is named after Hermann Kolbe.










