Cyclin G-associated kinase (GAK) is a serine/threonine kinase that in humans is encoded by the GAK gene. [5] [6]
Cyclin G-associated kinase (GAK) is a serine/threonine kinase that in humans is encoded by the GAK gene. [5] [6]
In all eukaryotes, the cell cycle is governed by cyclin-dependent protein kinases (CDKs), whose activities are regulated by cyclins and CDK inhibitors in a diverse array of mechanisms that involve the control of phosphorylation and dephosphorylation of Ser, Thr or Tyr residues. Cyclins are molecules that possess a consensus domain called the 'cyclin box.' In mammalian cells, 9 cyclin species have been identified, and they are referred to as cyclins A through I. Cyclin G is a direct transcriptional target of the p53 tumor suppressor gene product and thus functions downstream of p53. GAK is an association partner of cyclin G and CDK5. [5]
Cyclin G-associated kinase received its name because it immunoprecipitated with cyclin G though it now appears to not be associated with it. Cyclin G-associated kinase is homologous in function to the protein auxilin which when in association with Hsc70 uncoats clathrin in neuronal cells. However, the location of Cyclin G-associated kinase is not in the brain but near the trans-Golgi network of non-neuronal cells such as those found in the liver and testes. GAK is also known to be associated with focal adhesions though the exact relationship between the two is unknown. [7]
A structure of GAK was determined using X-ray diffraction to a resolution of 2.10 Å. [8]
Cyclin G-associated kinase is a two domain cystolic protein. The domain of interest is the C-terminal domain which consists of three subdomains such as a C-terminal J domain, a clathrin-binding domain, and a tension-like N-terminal domain. The other domain is the N terminal kinase domain which is a functional Ser/Thr protein kinase. The N-terminal kinase domain is able to phosphorylate histone H1. The subdomain tension-like N terminal function has not yet determined, though the domain shares high homology to the tumor suppressant PTEN. The key characteristic is the cysteine group which is required for phosphorylation; however the tension-like N terminal subdomain is absent of some important functional residues that PTEN has. The C-terminal J domain is responsible for the interaction with Hsc70, which is a molecular chaperone responsible for the uncoating of clathrin-coated vesicles during endocytosis. The clathrin-binding domain gathers clathrin into baskets. At pH 7 GAK allows Hsc70 to uncoat clathrin baskets and at pH 6 Hsc70 binds clathrin baskets without uncoating clathrin. Without taking into account GAK’s kinase domain, GAK is 43% identical to auxilin, a neuronal cell uncoating clathrin cofactor, in its amino acid composition. GAK is 57% homologous to auxilin if conserved residues are included in the comparison. Though similar domains of these two molecules suggest and have similar functions, the proteins carry these functions out in different manners. GAK initiates the assembly of clathrin baskets stoichiometrically, but at the different pHs it will either bind the Hsc70 to the baskets or induce the Hsc70 to uncoat the clathrin baskets catalytically which is one difference between auxilin and GAK. This catalytic route explains why less GAK than auxilin is needed to carry out a similar function. [7]
A protein phosphatase is a phosphatase enzyme that removes a phosphate group from the phosphorylated amino acid residue of its substrate protein. Protein phosphorylation is one of the most common forms of reversible protein posttranslational modification (PTM), with up to 30% of all proteins being phosphorylated at any given time. Protein kinases (PKs) are the effectors of phosphorylation and catalyse the transfer of a γ-phosphate from ATP to specific amino acids on proteins. Several hundred PKs exist in mammals and are classified into distinct super-families. Proteins are phosphorylated predominantly on Ser, Thr and Tyr residues, which account for 79.3, 16.9 and 3.8% respectively of the phosphoproteome, at least in mammals. In contrast, protein phosphatases (PPs) are the primary effectors of dephosphorylation and can be grouped into three main classes based on sequence, structure and catalytic function. The largest class of PPs is the phosphoprotein phosphatase (PPP) family comprising PP1, PP2A, PP2B, PP4, PP5, PP6 and PP7, and the protein phosphatase Mg2+- or Mn2+-dependent (PPM) family, composed primarily of PP2C. The protein Tyr phosphatase (PTP) super-family forms the second group, and the aspartate-based protein phosphatases the third. The protein pseudophosphatases form part of the larger phosphatase family, and in most cases are thought to be catalytically inert, instead functioning as phosphate-binding proteins, integrators of signalling or subcellular traps. Examples of membrane-spanning protein phosphatases containing both active (phosphatase) and inactive (pseudophosphatase) domains linked in tandem are known, conceptually similar to the kinase and pseudokinase domain polypeptide structure of the JAK pseudokinases. A complete comparative analysis of human phosphatases and pseudophosphatases has been completed by Manning and colleagues, forming a companion piece to the ground-breaking analysis of the human kinome, which encodes the complete set of ~536 human protein kinases.

Cyclin-dependent kinases (CDKs) are the families of protein kinases first discovered for their role in regulating the cell cycle. They are also involved in regulating transcription, mRNA processing, and the differentiation of nerve cells. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. In fact, yeast cells can proliferate normally when their CDK gene has been replaced with the homologous human gene. CDKs are relatively small proteins, with molecular weights ranging from 34 to 40 kDa, and contain little more than the kinase domain. By definition, a CDK binds a regulatory protein called a cyclin. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase but its activity can be typically further modulated by phosphorylation and other binding proteins, like p27. CDKs phosphorylate their substrates on serines and threonines, so they are serine-threonine kinases. The consensus sequence for the phosphorylation site in the amino acid sequence of a CDK substrate is [S/T*]PX[K/R], where S/T* is the phosphorylated serine or threonine, P is proline, X is any amino acid, K is lysine, and R is arginine.
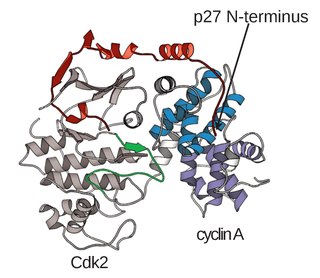
A cyclin-dependent kinase complex is a protein complex formed by the association of an inactive catalytic subunit of a protein kinase, cyclin-dependent kinase (CDK), with a regulatory subunit, cyclin. Once cyclin-dependent kinases bind to cyclin, the formed complex is in an activated state. Substrate specificity of the activated complex is mainly established by the associated cyclin within the complex. Activity of CDKCs is controlled by phosphorylation of target proteins, as well as binding of inhibitory proteins.
Maturation-promoting factor (abbreviated MPF, also called mitosis-promoting factor or M-Phase-promoting factor) is the cyclin-Cdk complex that was discovered first in frog eggs. It stimulates the mitotic and meiotic phases of the cell cycle. MPF promotes the entrance into mitosis (the M phase) from the G2 phase by phosphorylating multiple proteins needed during mitosis. MPF is activated at the end of G2 by a phosphatase, which removes an inhibitory phosphate group added earlier.

Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the HSPA8 gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging.

Cyclin-dependent kinase 2, also known as cell division protein kinase 2, or Cdk2, is an enzyme that in humans is encoded by the CDK2 gene. The protein encoded by this gene is a member of the cyclin-dependent kinase family of Ser/Thr protein kinases. This protein kinase is highly similar to the gene products of S. cerevisiae cdc28, and S. pombe cdc2, also known as Cdk1 in humans. It is a catalytic subunit of the cyclin-dependent kinase complex, whose activity is restricted to the G1-S phase of the cell cycle, where cells make proteins necessary for mitosis and replicate their DNA. This protein associates with and is regulated by the regulatory subunits of the complex including cyclin E or A. Cyclin E binds G1 phase Cdk2, which is required for the transition from G1 to S phase while binding with Cyclin A is required to progress through the S phase. Its activity is also regulated by phosphorylation. Multiple alternatively spliced variants and multiple transcription initiation sites of this gene have been reported. The role of this protein in G1-S transition has been recently questioned as cells lacking Cdk2 are reported to have no problem during this transition.

Cyclin-dependent kinase 4 also known as cell division protein kinase 4 is an enzyme that in humans is encoded by the CDK4 gene. CDK4 is a member of the cyclin-dependent kinase family.

Cell division protein kinase 6 (CDK6) is an enzyme encoded by the CDK6 gene. It is regulated by cyclins, more specifically by Cyclin D proteins and Cyclin-dependent kinase inhibitor proteins. The protein encoded by this gene is a member of the cyclin-dependent kinase, (CDK) family, which includes CDK4. CDK family members are highly similar to the gene products of Saccharomyces cerevisiae cdc28, and Schizosaccharomyces pombe cdc2, and are known to be important regulators of cell cycle progression in the point of regulation named R or restriction point.

A cyclin-dependent kinase inhibitor protein

Cyclin-dependent kinase 9 or CDK9 is a cyclin-dependent kinase associated with P-TEFb.

Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast S. cerevisiae, and the fission yeast S. pombe, where it is encoded by genes cdc28 and cdc2, respectively. With its cyclin partners, Cdk1 forms complexes that phosphorylate a variety of target substrates ; phosphorylation of these proteins leads to cell cycle progression.

Cyclin-dependent kinase 7, or cell division protein kinase 7, is an enzyme that in humans is encoded by the CDK7 gene.

M-phase inducer phosphatase 1 also known as dual specificity phosphatase Cdc25A is a protein that in humans is encoded by the cell division cycle 25 homolog A (CDC25A) gene.

G1/S-specific cyclin-E1 is a protein that in humans is encoded by the CCNE1 gene.

Cyclin-A1 is a protein that in humans is encoded by the CCNA1 gene.

Cyclin-dependent kinase 4 inhibitor D is an enzyme that in humans is encoded by the CDKN2D gene.

Cyclin-dependent kinase inhibitor 3 is an enzyme that in humans is encoded by the CDKN3 gene.

Cell division protein kinase 10 is an enzyme that in humans is encoded by the CDK10 gene.

Cyclin-K is a protein that in humans is encoded by the CCNK gene.

Cyclin-dependent kinase 5 is a protein, and more specifically an enzyme, that is encoded by the Cdk5 gene. It was discovered 15 years ago, and it is saliently expressed in post-mitotic central nervous system neurons (CNS).
This article incorporates text from the United States National Library of Medicine, which is in the public domain.