In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air.

Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis.

Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Hypofluorous acid, chemical formula HOF, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in this acid is 0, while its valence is 2. It is also the only hypohalous acid that can be isolated as a solid. HOF is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride, oxygen difluoride, hydrogen peroxide, ozone and oxygen. HOF is explosive at room temperature, forming HF and O2:

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:
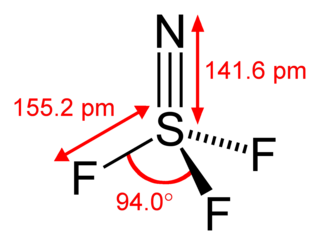
Thiazyl trifluoride is a chemical compound of nitrogen, sulfur, and fluorine, having the formula NSF3. It exists as a stable, colourless gas, and is an important precursor to other sulfur-nitrogen-fluorine compounds. It has tetrahedral molecular geometry around the sulfur atom, and is regarded to be a prime example of a compound that has a sulfur-nitrogen triple bond.

Mercury(IV) fluoride, HgF4, is a purported compound, the first to be reported with mercury in the +4 oxidation state. Mercury, like the other group 12 elements (cadmium and zinc), has an s2d10 electron configuration and generally only forms bonds involving its 6s orbital. This means that the highest oxidation state mercury normally attains is +2, and for this reason it is sometimes considered a post-transition metal instead of a transition metal. HgF4 was first reported from experiments in 2007, but its existence remains disputed; experiments conducted in 2008 could not replicate the compound.

Mercury(I) fluoride or mercurous fluoride is the chemical compound composed of mercury and fluorine with the formula Hg2F2. It consists of small yellow cubic crystals, which turn black when exposed to light.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Radon compounds are chemical compounds formed by the element radon (Rn). Radon is a noble gas, i.e. a zero-valence element, and is chemically not very reactive. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas under normal circumstances, and its decay-chain parents are not, it can readily be extracted from them for research.