
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.

In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses such as change in the electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway.

A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins. They are sometimes called blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists have affinity but no efficacy for their cognate receptors, and binding will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors. Antagonists mediate their effects by binding to the active site or to the allosteric site on a receptor, or they may interact at unique binding sites not normally involved in the biological regulation of the receptor's activity. Antagonist activity may be reversible or irreversible depending on the longevity of the antagonist–receptor complex, which, in turn, depends on the nature of antagonist–receptor binding. The majority of drug antagonists achieve their potency by competing with endogenous ligands or substrates at structurally defined binding sites on receptors.

WIN 35,428 is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
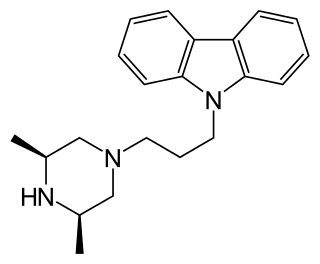
Rimcazole is an antagonist of the sigma receptor as well as a dopamine reuptake inhibitor. Sigma receptors are thought to be involved in the drug psychosis that can be induced by some drugs such as phencyclidine and cocaine, and rimcazole was originally researched as a potential antipsychotic with a different mechanism of action to traditional antipsychotic drugs. Trials proved inconclusive and rimcazole was not pursued for this application, but other sigma antagonists continue to be researched for a variety of potential applications. Rimcazole has been shown to reduce the effects of cocaine, and analogues of rimcazole have been shown to be highly effective at blocking the convulsions caused by cocaine overdose in animal models.
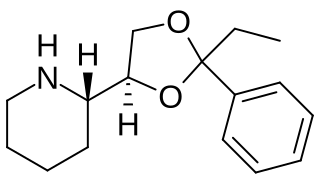
Etoxadrol (CL-1848C) is a dissociative anaesthetic drug that has been found to be an NMDA antagonist and produce similar effects to PCP in animals. Etoxadrol, along with another related drug dexoxadrol, were developed as analgesics for use in humans, but development was discontinued in the late 1970s after patients reported side effects such as nightmares and hallucinations.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

2-Benzylpiperidine is a stimulant drug of the piperidine class. It is similar in structure to other drugs such as methylphenidate and desoxypipradrol but around one twentieth as potent, and while it boosts norepinephrine levels to around the same extent as d-amphetamine, it has very little effect on dopamine levels, with its binding affinity for the dopamine transporter around 175 times lower than for the noradrenaline transporter. 2-benzylpiperidine is little used as a stimulant, with its main use being as a synthetic intermediate in the manufacture of other drugs.

RTI-126 is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter. RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.
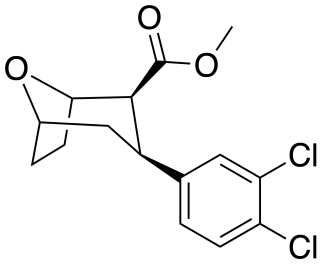
Tropoxane (O-1072) is an aryloxytropane derivative drug developed by Organix Inc., which acts as a stimulant and potent dopamine and serotonin reuptake inhibitor. It is an analogue of dichloropane where the amine nitrogen has been replaced by an oxygen ether link, demonstrating that the amine nitrogen is not required for DAT binding and reuptake inhibition.

Salicylmethylecgonine, (2′-Hydroxycocaine) is a tropane derivative drug which is both a synthetic analogue and a possible active metabolite of cocaine. Its potency in vitro is around 10x that of cocaine, although it is only around three times more potent than cocaine when administered to mice Note however that the compound 2′-Acetoxycocaine would act as a prodrug to Salicylmethylecgonine in humans, and has a more efficient partition coefficient which would act as a delivery system and would circumvent this reason for a drop in potency. Salicylmethylecgonine also shows increased behavioral stimulation compared to cocaine similar to the phenyltropanes. The hydroxy branch renders the molecule a QSAR of a 10-fold increase over cocaine in its binding potency for the dopamine transporter & a 52-fold enhanced affinity for the norepinephrine transporter. It also has a reduced selectivity for the serotonin transporter though only due to its greater increase at NET binding; its SERT affinity being 4-fold increased compared to cocaine. However, in overall binding affinity it displaces ligands better across the board than cocaine in all monoamine categories.

RTI-229, also known as (–)-3β-(4-iodophenyl)tropane-2β-pyrrolidine carboxamide and RTI-4229-229, is a potent and long-lasting stimulant drug which was developed in the 1990s as part of a large group of related analogues from the phenyltropane family. With the combination of two potent dopamine transporter (DAT) binding motifs attached to the tropane ring, the p-iodophenyl group at the 3β-position and a pyrrolidine carboxamide at 2β, RTI-229 has extremely high selectivity for the dopamine transporter and is one of the most DAT-selective compounds in the RTI series.

Metaphit is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the m-isothiocyanate derivative of phencyclidine (PCP) and binds irreversibly to the PCP binding site on the NMDA receptor complex. However, later studies suggest the functionality of metaphit is mediated by sites not involved in PCP-induced passive avoidance deficit, and not related to the NMDA receptor complex. Metaphit was also shown to prevent d-amphetamine induced hyperactivity, while significantly depleting dopamine content in the nucleus accumbens. Metaphit was the first acylating ligand used to study the cocaine receptor. It is a structural isomer of the similar research compound fourphit, as it and metaphit both are isothiocyanate substituted derivatives of an analogous scaffold shared with PCP.

4-Fluoromethylphenidate is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.
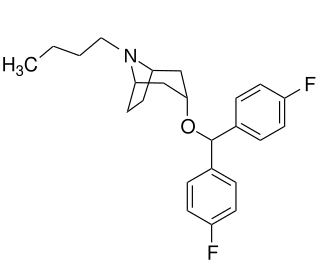
JHW-007 is a cocaine analogue and a high affinity atypical dopamine reuptake inhibitor that is being researched for the treatment of cocaine addiction. JHW-007 has been found to blunt the psychostimulant effects of cocaine and reduce self-administration in rodents. JHW-007 exposure has been shown to block the conditioned place preference effects of cocaine. JHW-007 may directly antagonize the autoregulatory dopamine D2 receptor, a hypothesis that was developed following the observation of JHW-007's ability to inhibit D2 receptor-mediated currents in the midbrain.


















