Diphosgene is an organic chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.
Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug oseltamivir marketed by Hoffmann-La Roche under the trade name Tamiflu. Its commercial production starts from the biomolecule shikimic acid harvested from Chinese star anise and from recombinant E. coli. Control of stereochemistry is important: the molecule has three stereocenters and the sought-after isomer is only 1 of 8 stereoisomers.
{{Infobox scientist | name = Max Bergmann | image = | caption = | birth_date = 12 February 1886 | birth_place = Fürth, Germany | nationality = | death_date = 7 November 1944 (aged 58) | death_place = New York City, United States | field = peptide chemistry | work_institution = Kaiser Wilhelm Institute for Leather Research
Rockefeller Institute for Medical Research | alma_mater = Ludwig Maximilian University of Munich, Friedrich Wilhelm University | doctoral_advisor = Ignaz Bloch | doctoral_students = Leonidas Zervas | known_for = Carboxybenzyl protecting group | spouse = Emmy Bergmann [[:de:Emmy Bergmann | children = Peter Bergmann (physicist) }} Max Bergmann was a Jewish-German biochemist. Together with Leonidas Zervas, the discoverer of the group, they were the first to use the carboxybenzyl protecting group for the synthesis of oligopeptides.

The Bergmann azlactone peptide synthesis is a classic organic synthesis process for the preparation of dipeptides.
The Bergmann degradation is a series of chemical reactions designed to remove a single amino acid from the carboxylic acid (C-terminal) end of a peptide. First demonstrated by Max Bergmann in 1934, it is a rarely used method for sequencing peptides. The later developed Edman degradation is an improvement upon the Bergmann degradation, instead cleaving the N-terminal amino acid of peptides to produce a hydantoin containing the desired amino acid.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.

The Achmatowicz reaction, also known as the Achmatowicz rearrangement, is an organic synthesis in which a furan is converted to a dihydropyran. In the original publication by the Polish Chemist Osman Achmatowicz Jr. in 1971 furfuryl alcohol is reacted with bromine in methanol to 2,5-dimethoxy-2,5-dihydrofuran which rearranges to the dihydropyran with dilute sulfuric acid. Additional reaction steps, alcohol protection with methyl orthoformate and boron trifluoride) and then ketone reduction with sodium borohydride produce an intermediate from which many monosaccharides can be synthesised.
Chloroformic acid is a chemical compound with the formula ClCO2H. It is the single acyl-halide derivative of carbonic acid. Chloroformic acid is also structurally related to formic acid, in a way that the non-acidic hydrogen of formic acid is replaced by chlorine. Despite the similar name, it is very different from chloroform. It is described as unstable.

Chloroformates are a class of organic compounds with the formula ROC(O)Cl. They are formally esters of chloroformic acid. Most are colorless, volatile liquids that degrade in moist air. A simple example is methyl chloroformate, which is commercially available.

Trichloroethyl chloroformate is used in organic synthesis for the introduction of the trichloroethyl chloroformate (Troc) protecting group for amines, thiols and alcohols. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy.

Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis.
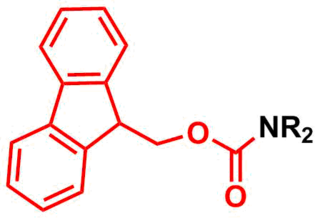
The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile protecting group used in organic synthesis.
Leonidas Zervas was a Greek organic chemist who made seminal contributions in peptide chemical synthesis. Together with his mentor Max Bergmann they laid the foundations for the field in 1932 with their major discovery, the Bergmann-Zervas carboxybenzoxy oligopeptide synthesis which remained unsurpassed in utility for the next two decades. The carboxybenzyl protecting group he discovered is often abbreviated Z in his honour.
Iphigenia Photaki was a Greek organic chemist remembered for her contributions in peptide chemical synthesis, especially in the synthesis of biologically/enzymatically active peptides.