
Catechol-O-methyltransferase is one of several enzymes that degrade catecholamines, catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-O-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957.

l-DOPA, also known as l-3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine.
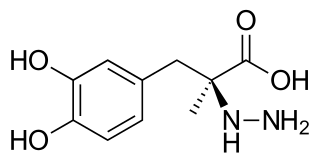
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of administered levodopa to cross the blood–brain barrier for central nervous system effect, instead of being peripherally metabolised into substances unable to cross said barrier.
Dyskinesia refers to a category of movement disorders that are characterized by involuntary muscle movements, including movements similar to tics or chorea and diminished voluntary movements. Dyskinesia can be anything from a slight tremor of the hands to an uncontrollable movement of the upper body or lower extremities. Discoordination can also occur internally especially with the respiratory muscles and it often goes unrecognized. Dyskinesia is a symptom of several medical disorders that are distinguished by their underlying cause.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.
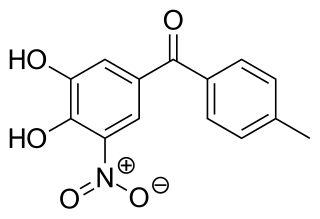
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.

d-DOPA(D-3,4-dihydroxyphenylalanine;dextrodopa) is similar to L-DOPA (levodopa), but with opposite chirality. Levo- and dextro- rotation refer to a molecule's ability to rotate planes of polarized light in one or the other direction. Whereas L-DOPA is moderately effective in the treatment of Parkinson's disease (PD) and dopamine-responsive dystonia (DRD) by stimulating the production of dopamine in the brain, D-DOPA is biologically inactive.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.

A catechol-O-methyltransferase inhibitor is a drug that inhibits the enzyme catechol-O-methyltransferase. This enzyme methylates catecholamines such as dopamine, norepinephrine and epinephrine. It also methylates levodopa. COMT inhibitors are indicated for the treatment of Parkinson's disease in combination with levodopa and an aromatic L-amino acid decarboxylase inhibitor. The therapeutic benefit of using a COMT inhibitor is based on its ability to prevent the methylation of levodopa to 3-O-methyldopa, thus increasing the bioavailability of levodopa. COMT inhibitors significantly decrease off time in people with Parkinson's disease also taking carbidopa/levodopa.

Droxidopa, also known as L-threo-dihydroxyphenylserine (L-DOPS) and sold under the brand names Northera and Dops among others, is sympathomimetic medication which is used in the treatment of hypotension and for other indications. It is taken by mouth.

Levodopa, also known as L-DOPA and sold under many brand names, is a dopaminergic medication which is used in the treatment of Parkinson's disease and certain other conditions like dopamine-responsive dystonia and restless legs syndrome. The drug is usually used and formulated in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor like carbidopa or benserazide. Levodopa is taken by mouth, by inhalation, through an intestinal tube, or by administration into fat.

Dopamine dysregulation syndrome (DDS) is a dysfunction of the reward system observed in some individuals taking dopaminergic medications for an extended length of time. It typically occurs in people with Parkinson's disease (PD) who have taken dopamine agonist medications for an extended period of time. It is characterized by problems such as addiction to medication, gambling, or sexual behavior.

A-86929 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. It was developed as a possible treatment for Parkinson's disease, as well as for other applications such as treatment of cocaine addiction, but while it had reasonable efficacy in humans it also caused dyskinesias and has not been continued. It has mainly been used as its diacetate ester prodrug adrogolide (ABT-431), which has better bioavailability.
Levodopa-induced dyskinesia (LID) is a form of dyskinesia associated with levodopa (l-DOPA), used to treat Parkinson's disease. It often involves hyperkinetic movements, including chorea, dystonia, and athetosis.

Monoamine precursors are precursors of monoamines and monoamine neurotransmitters in the body. The amino acids L-tryptophan and L-5-hydroxytryptophan are precursors of serotonin and melatonin, while the amino acids L-phenylalanine, L-tyrosine, and L-DOPA (levodopa) are precursors of dopamine, epinephrine (adrenaline), and norepinephrine (noradrenaline).
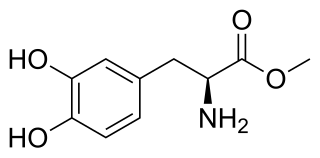
Melevodopa/carbidopa, sold under the brand name Sirio, is a combination of melevodopa, a prodrug of the dopamine precursor and hence non-selective dopamine receptor agonist levodopa (L-DOPA), and carbidopa, a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor, which is used in the treatment of Parkinson's disease in Italy. It is taken orally in the form of tablets.
XP-21279 is a sustained-release levodopa (L-DOPA) prodrug and hence a dopamine precursor and non-selective dopamine receptor agonist which was under development for the treatment of Parkinson's disease. It is taken by mouth.

O,O′-Diacetyldopamine, or 3,4-O-diacetyldopamine, also known as 3,4-diacetoxyphenethylamine, is a synthetic derivative of dopamine in which both of the hydroxyl groups have been acetylated.

DA-Phen, also known as dopamine–phenylalanine conjugate, is a synthetic dopamine prodrug which is under preclinical evaluation. Dopamine itself is hydrophilic and is unable to cross the blood–brain barrier, thus showing peripheral selectivity. DA-Phen was developed as a dopamine prodrug that would allow for entry into the central nervous system via passive diffusion and/or active transport.

O,O′-Dipivaloyldopamine, or simply dipivaloyldopamine, also known as 3,4-pivaloyloxyphenethylamine, is a synthetic derivative of dopamine in which both of the hydroxyl groups have been acetylated. It was developed as a lipophilic prodrug of dopamine that would allow for entry of dopamine into the central nervous system.















