
Phenylpropanolamine (PPA), sold under many brand names, is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was previously commonly used in prescription and over-the-counter cough and cold preparations. The medication is taken by mouth.

l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with designing and developing pharmaceutical drugs. Medicinal chemistry involves the identification, synthesis and development of new chemical entities suitable for therapeutic use. It also includes the study of existing drugs, their biological properties, and their quantitative structure-activity relationships (QSAR).
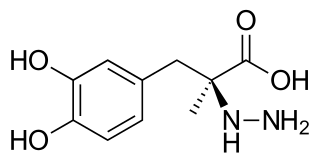
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of administered levodopa to cross the blood–brain barrier for central nervous system effect, instead of being peripherally metabolised into substances unable to cross said barrier.
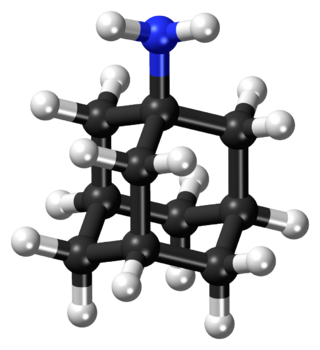
Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended because of widespread drug resistance. It is also used for a variety of other uses. The drug is taken by mouth.

Orphenadrine is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is a muscle relaxant that is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs. It is considered a dirty drug due to its multiple mechanisms of action in different pathways. It was discovered and developed in the 1940s.

Dopaminergic means "related to dopamine", dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Apomorphine, sold under the brand name Apokyn among others, is a type of aporphine having activity as a non-selective dopamine agonist which activates both D2-like and, to a much lesser extent, D1-like receptors. It also acts as an antagonist of 5-HT2 and α-adrenergic receptors with high affinity. The compound is historically a morphine decomposition product made by boiling morphine with concentrated acid, hence the -morphine suffix. Contrary to its name, apomorphine does not actually contain morphine or its skeleton, nor does it bind to opioid receptors. The apo- prefix relates to it being a morphine derivative ("[comes] from morphine").

A dopamine agonist is a compound that activates dopamine receptors. There are two families of dopamine receptors, D1-like and D2-like. They are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used in the treatment of the motor symptoms of Parkinson's disease, and to a lesser extent, in hyperprolactinemia and restless legs syndrome. They are also used off-label in the treatment of clinical depression. Impulse control disorders are associated with the use of dopamine agonists for whatever condition.
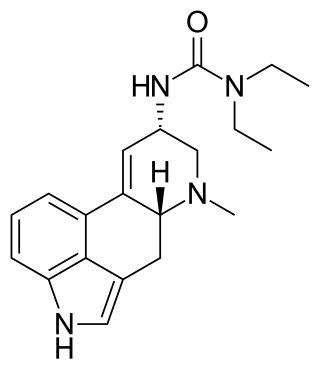
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

Piribedil (trade names Pronoran, Trivastal Retard, Trastal, Trivastan, Clarium and others) is an antiparkinsonian agent and piperazine derivative which acts as a D2 and D3 receptor agonist. It also has α2-adrenergic antagonist properties.

Dihydroergocryptine (DHEC), sold under the brand names Almirid and Cripar among others, is a dopamine agonist of the ergoline group that is used as an antiparkinson agent in the treatment of Parkinson's disease. It is taken by mouth.

Budipine is an antiparkinson agent marketed for the treatment of Parkinson's disease.

Safinamide is a drug used as an add-on treatment for Parkinson's disease with "off" episodes; it has multiple modes of action, including the inhibition of monoamine oxidase B.

Bromantane, sold under the brand name Ladasten, is an atypical psychostimulant and anxiolytic drug of the adamantane family related to amantadine and memantine which is used in Russia in the treatment of neurasthenia. Although the effects of bromantane have been determined to be dependent on the dopaminergic and possibly serotonergic neurotransmitter systems, its exact mechanism of action is unknown, and it is distinct in its properties relative to typical psychostimulants such as amphetamine. Bromantane has sometimes been described instead as an actoprotector.
Levodopa-induced dyskinesia (LID) is a form of dyskinesia associated with levodopa (l-DOPA), used to treat Parkinson's disease. It often involves hyperkinetic movements, including chorea, dystonia, and athetosis.

Emoxypine (2-ethyl-6-methyl-3-hydroxypyridine), also known as Mexidol or Mexifin, a succinate salt, is chemical compound which is claimed by its manufacturer, the Russian company Pharmasoft Pharmaceuticals, to have antioxidant and actoprotector properties. Its chemical structure resembles that of pyridoxine (a type of vitamin B6).
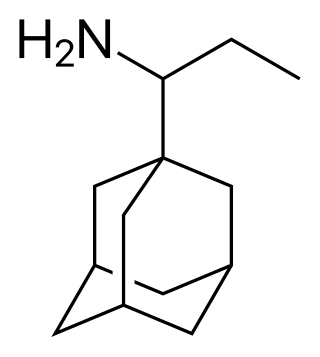
Adapromine is an antiviral drug of the adamantane group related to amantadine (1-aminoadamantane), rimantadine, and memantine (1-amino-3,5-dimethyladamantane) that is marketed in Russia for the treatment and prevention of influenza. It is an alkyl analogue of rimantadine and is similar to rimantadine in its antiviral activity but possesses a broader spectrum of action, being effective against influenza viruses of both type A and B. Strains of type A influenza virus with resistance to adapromine and rimantadine and the related drug deitiforine were encountered in Mongolia and the Soviet Union in the 1980s.
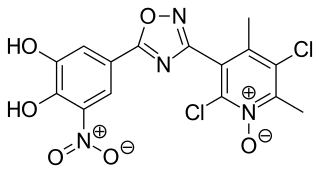
Opicapone, sold under the brand name Ongentys, is a medication which is administered together with levodopa in people with Parkinson's disease. Opicapone is a catechol-O-methyltransferase (COMT) inhibitor.

Flumezapine is an abandoned, investigational antipsychotic drug that was studied for the treatment of schizophrenia. Flumezapine failed clinical trials due to concern for liver and muscle toxicity. Flumezapine is structurally related to the common antipsychotic olanzapine—a point that was used against its manufacturer, Eli Lilly and Company, in a lawsuit in which generic manufacturers sought to void the patent on brand name olanzapine (Zyprexa). Although flumezapine does not differ greatly from olanzapine in terms of its structure, the difference was considered to be non-obvious, and Eli Lilly's patent rights on Zyprexa were upheld.



















