Related Research Articles

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Accurate regulation of GABAergic transmission through appropriate developmental processes, specificity to neural cell types, and responsiveness to activity is crucial for the proper functioning of nearly all aspects of the central nervous system (CNS). Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions and, to a lesser extent, bicarbonate ions.
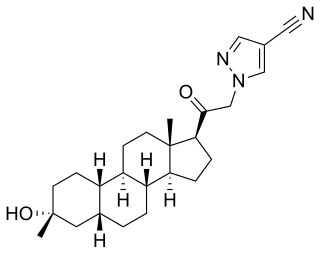
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Etifoxine, sold under the trade name Stresam among others, is a nonbenzodiazepine anxiolytic agent, primarily indicated for short-term management of adjustment disorder, specifically instances of situational depression accompanied by anxiety, such as stress-induced anxiety. Administration is by mouth. Side effects associated with etifoxine use include slight drowsiness, headache, skin eruptions, and allergic reactions. In rare cases, etifoxine has been linked to severe skin and liver toxicity, as well as menstrual bleeding between periods. Unlike benzodiazepines, etifoxine does not cause sedation or lack of coordination. Etifoxine acts as a GABAA receptor positive allosteric modulator and as a ligand for translocator proteins. Both mechanisms are conjectured to contribute to its anxiolytic properties.
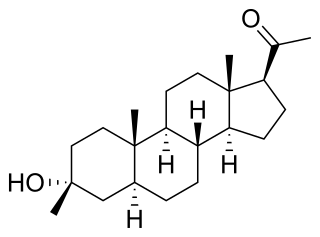
Ganaxolone, sold under the brand name Ztalmy, is a medication used to treat seizures in people with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder. Ganaxolone is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

Fasedienol, also known as 4-androstadienol or as 4,16-androstadien-3β-ol, is a pherine which is under development by VistaGen Therapeutics in a nasal spray formulation (PRN) for the acute treatment of social anxiety disorder. It is also being investigated by VistaGen Therapeutics for the treatment of generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD). The pherine is a positional isomer of the endogenous pheromone androstadienol. As of 2020, it is in phase III clinical trials for social anxiety disorder.
A neurosteroidogenesis inhibitor is a drug that inhibits the production of endogenous neurosteroids. Neurosteroids include the excitatory neurosteroids pregnenolone sulfate, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S), and the inhibitory neurosteroids allopregnanolone, tetrahydrodeoxycorticosterone (THDOC), and 3α-androstanediol, among others. By inhibiting the synthesis of endogenous neurosteroids, neurosteroidogenesis inhibitors have effects in the central nervous system.

Tulrampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by RespireRx Pharmaceuticals and Servier for the treatment of major depressive disorder, Alzheimer's disease, dementia, and mild cognitive impairment. Tulrampator was in phase II clinical trial for depression, but failed to show superiority over placebo. There are also phase II clinical trials for Alzheimer's disease and phase I trials for dementia and mild cognitive impairment.

Zuranolone, sold under the brand name Zurzuvae, is a medication used for the treatment of postpartum depression. It is taken by mouth.
A GABAA receptor negative allosteric modulator is a negative allosteric modulator (NAM), or inhibitor, of the GABAA receptor, a ligand-gated ion channel of the major inhibitory neurotransmitter γ-aminobutyric acid (GABA). They are closely related and similar to GABAA receptor antagonists. The effects of GABAA receptor NAMs are functionally the opposite of those of GABAA receptor positive allosteric modulators (PAMs) like the benzodiazepines, barbiturates, and ethanol (alcohol). Non-selective GABAA receptor NAMs can produce a variety of effects including convulsions, neurotoxicity, and anxiety, among others.

GL-II-73 (GL-ii-073) is a benzodiazepine derivative related in chemical structure to compounds such as midazolam and adinazolam. It is described as an α5 preferring positive allosteric modulator of the benzodiazepine site of GABAA receptors, with weaker activity at α2 and α3 and no significant affinity for the α1 subtype. In animal tests it was found to produce effects consistent with antidepressant, anxiolytic and nootropic actions.

Posovolone is a synthetic neurosteroid which was under development as a sedative/hypnotic medication for the treatment of insomnia. It is orally active and acts as a GABAA receptor positive allosteric modulator. In animals, posovolone shows anticonvulsant, anxiolytic-like, ataxic, and sleep-promoting effects and appeared to produce effects similar to those of pregnanolone. Development of the agent was started by 1999 and appears to have been discontinued by 2007. In 2021, an INNTooltip International Nonproprietary Name was registered for posovolone with the descriptor of "antidepressant". Posovolone was originally developed by Purdue Pharma.
Deuterated etifoxine is a deuterated drug which is under development for the treatment of anxiety disorders and mood disorders.
References
- 1 2 3 4 5 6 7 8 9 Cutler AJ, Mattingly GW, Maletic V (June 2023). "Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder". Transl Psychiatry. 13 (1): 228. doi:10.1038/s41398-023-02514-2. PMC 10293235 . PMID 37365161.
- 1 2 3 4 5 6 7 8 9 "PRAX 114". AdisInsight. Springer Nature Switzerland AG. 28 March 2023. Retrieved 21 October 2024.
- 1 2 3 4 "Delving into the Latest Updates on PRAX-114 with Synapse". Synapse. 19 October 2024. Retrieved 21 October 2024.
- 1 2 3 4 5 Scala M, Fanelli G, De Ronchi D, Serretti A, Fabbri C (September 2023). "Clinical specificity profile for novel rapid acting antidepressant drugs". Int Clin Psychopharmacol. 38 (5): 297–328. doi:10.1097/YIC.0000000000000488. PMC 10373854 . PMID 37381161.
- 1 2 3 4 Hughes Z, Scott L, Kahlig K, Wittmann M (2021). "PRAX-114 is a Novel Extrasynaptic GABA-A Receptor Preferring Positive Allosteric Modulator With a Wide Separation Between a Translational Biomarker Signature Associated With Antidepressant-Like Activity, and Sedative Effects". Biological Psychiatry. 89 (9). Elsevier BV: S204. doi:10.1016/j.biopsych.2021.02.517. ISSN 0006-3223.
- 1 2 3 Hughes Z, Scott L, Kahlig K, Wittmann M (2022). "P140. Preference for Extrasynaptic GABAA Receptors Conveys a Wider Therapeutic Window Between Anxiolytic and Sedative-Like Effects in Rats for the Positive Allosteric Modulator, PRAX-114, Compared With Zuranolone". Biological Psychiatry. 91 (9). Elsevier BV: S143–S144. doi:10.1016/j.biopsych.2022.02.374. ISSN 0006-3223.