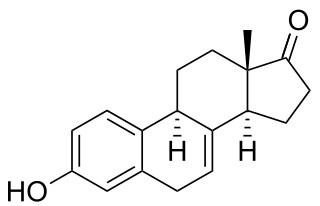
Equilin is a naturally occurring estrogen sex hormone found in horses as well as a medication. It is one of the estrogens present in the estrogen combination drug preparations known as conjugated estrogens and esterified estrogens. CEEs is the most commonly used form of estrogen medications in hormone replacement therapy (HRT) for menopausal symptoms in the United States. Estrone sulfate is the major estrogen in CEEs while equilin sulfate is the second major estrogen in the formulation, present as about 25% of the total.

Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disorders, and certain hormone-sensitive cancers. It is usually taken by mouth but is also used as a patch and vaginal ring.
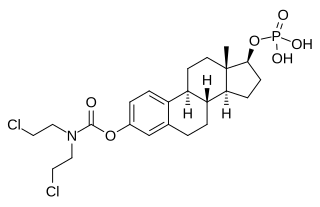
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Ethinylestradiol sulfonate (EES), sold under the brand names Deposiston and Turisteron among others, is an estrogen medication which has been used in birth control pills for women and in the treatment of prostate cancer in men. It has also been investigated in the treatment of breast cancer in women. The medication was combined with norethisterone acetate in birth control pills. EES is taken by mouth once per week.

A steroid ester is an ester of a steroid. They include androgen esters, estrogen esters, progestogen esters, and corticosteroid esters. Steroid esters may be naturally occurring/endogenous like DHEA sulfate or synthetic like estradiol valerate. Esterification is useful because it is often able to render the parent steroid into a prodrug of itself with altered chemical properties such as improved metabolic stability, water solubility, and/or lipophilicity. This, in turn, can enhance pharmacokinetics, for instance by improving the steroid's bioavailability and/or conferring depot activity and hence an extended duration with intramuscular or subcutaneous injection.

Irosustat is an orally active, irreversible, nonsteroidal inhibitor of steroid sulfatase (STS) and member of the aryl sulfamate ester class of drugs that was under development by Sterix Ltd and Ipsen for the treatment of hormone-sensitive cancers such as breast cancer, prostate cancer, and endometrial cancer but has not yet been marketed. The drug was first designed and synthesized in the group of Professor Barry V L Potter at the Department of Pharmacy & Pharmacology, University of Bath, working together with Professor Michael J. Reed at Imperial College, London and its initial development was undertaken through the university spin-out company Sterix Ltd and overseen by Cancer Research UK (CRUK). Results of the "first-in-class" clinical trial in breast cancer of an STS inhibitor in humans were published in 2006 and dose optimisation studies and further clinical data have been reported.

Estradiol sulfamate, or estradiol-3-O-sulfamate, is a steroid sulfatase (STS) inhibitor which is under development for the treatment of endometriosis. It is the C3 sulfamate ester of estradiol, and was originally thought to be a prodrug of estradiol.

Estrone sulfamate, or estrone-3-O-sulfamate, is a steroid sulfatase (STS) inhibitor which has not yet been marketed. It is the C3 sulfamate ester of the estrogen estrone. Unlike other estrogen esters however, EMATE is not an effective prodrug of estrogens. A closely related compound is estradiol sulfamate (E2MATE), which is extensively metabolized into EMATE and has similar properties to it.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.
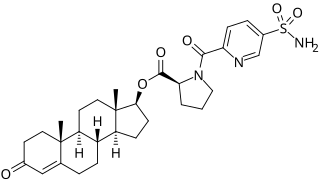
EC586, also known as testosterone 17β-(1- -L-proline), is an androgen and anabolic steroid which is under development by Evestra for use in androgen replacement therapy in men. It is an orally active androgen ester – specifically, a C17β sulfonamide–proline ester of the natural and bioidentical androgen testosterone – and acts as a prodrug of testosterone in the body. However, unlike oral testosterone and conventional oral testosterone esters such as testosterone undecanoate, EC586 has high oral potency, may undergo little or no first-pass metabolism, and may not have disproportionate androgenic effects in the liver. As such, it may have a variety of desirable advantages over oral testosterone, similarly to parenteral testosterone, but with the convenience of oral administration. Evestra intends to seek Investigational New Drug status for EC586 in the fourth quarter of 2018.

Estriol sulfamate, or estriol 3-O-sulfamate, is a synthetic estrogen and estrogen ester which was never marketed. It is the C3 sulfamate ester of estriol. The drug shows substantially improved oral estrogenic potency relative to estriol in rats but without an increase in hepatic estrogenic potency. However, the closely related compound estradiol sulfamate (E2MATE) failed to show estrogenic activity in humans, which is due to the fact that it is additionally a highly potent inhibitor of steroid sulfatase which regulates the estrogenicity of such compounds and thus it prevents its own bioactivation into estradiol.

Ethinylestradiol sulfamate, or 17α-ethynylestradiol 3-O-sulfamate, is a synthetic estrogen and estrogen ester which was never marketed. It is the C3 sulfamate ester of ethinylestradiol. The drug shows considerably improved oral estrogenic potency (uterotrophic) relative to ethinylestradiol in rats but without an increase in hepatic estrogenic potency. Related compounds like ethinylestradiol N,N-diethylsulfamate (J271) and ethinylestradiol pyrrolidinosulfonate (J272) have also been developed, and have similar properties in animals. However, the closely related compound estradiol sulfamate (E2MATE) failed to show estrogenic activity in humans, which is due to the fact that it is additionally a highly potent inhibitor of steroid sulfatase and prevents its own bioactivation into estradiol.

Ethinylestradiol sulfate, also known as 17α-ethynylestradiol 3-sulfate, is an estrogen ester – specifically, the C3 sulfuric acid (sulfate) ester of the synthetic estrogen ethinylestradiol (EE) – and is the major metabolite of EE. Circulating levels of EE sulfate range from 6 to 22 times those of EE when EE is taken orally. EE sulfate can be transformed back into EE (14–21%) via steroid sulfatase, and it has been suggested that EE sulfate may serve as a circulating reservoir for EE, similarly to the case of estrone sulfate with estradiol. However, the EE sulfate pool with EE is far smaller than the pool of estrone sulfate that occurs with estradiol. In addition, in contrast to the case of estrone sulfate and estrone, the conversion rate of EE sulfate back into EE is relatively low, and has been said probably isn't of clinical significance. However, other studies have suggested that EE sulfate may nonetheless contribute up to 20% of total EE levels.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.















