
Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. It is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender people, and in hormonal birth control. It is also used in the treatment of prostate cancer. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.
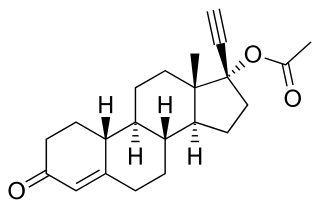
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders. It is also used in the treatment of prostate cancer in men. Estradiol benzoate is used in veterinary medicine as well. When used clinically, the medication is given by injection into muscle usually two to three times per week.

Estradiol cypionate (EC), sold under the brand name Depo-Estradiol among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for trans women, and in hormonal birth control for women. It is given by injection into muscle once every 1 to 4 weeks.

Estradiol undecylate, also known as estradiol undecanoate and formerly sold under the brand names Delestrec and Progynon Depot 100 among others, is an estrogen medication which has been used in the treatment of prostate cancer in men. It has also been used as a part of hormone therapy for transgender women. Although estradiol undecylate has been used in the past, it was discontinued .The medication has been given by injection into muscle usually once a month.
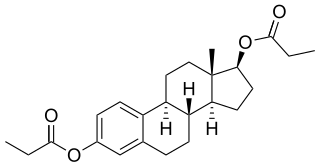
Estradiol dipropionate (EDP), sold under the brand names Agofollin, Di-Ovocylin, and Progynon DP among others, is an estrogen medication which has been used in hormone therapy for menopausal symptoms and low estrogen levels in women and in the treatment of gynecological disorders. It has also been used in feminizing hormone therapy for transgender women and in the treatment of prostate cancer in men. Although widely used in the past, estradiol dipropionate has largely been discontinued and is mostly no longer available today. It appears to remain in use only in Japan, Macedonia, and Australia. Estradiol dipropionate is given by injection into muscle at intervals ranging from once or twice a week to once every week and a half to two weeks.

Estradiol hexahydrobenzoate (EHHB), sold under a number of brand names including Benzo-Ginoestril A.P., BenzoGynoestryl Retard, Ginestryl-15-Depot, Menodin, and Tardoginestryl, is an estrogen medication which was previously used for indications such as menopausal hormone therapy and gynecological disorders. EHHB is given by injection into muscle at regular intervals, for instance once every few weeks.

Estradiol phenylpropionate (EPP), also known as estradiol 17β-phenylpropionate and sold under the brand name Menformon Prolongatum, is an estrogen which is no longer marketed. It is an estrogen ester, specifically the C17β phenylpropionate ester of estradiol.

Estradiol dienanthate (EDE), sold under the brand names Climacteron among others, is a long-acting estrogen medication which was previously used in menopausal hormone therapy for women and to suppress lactation in women. It was formulated in combination with estradiol benzoate (EB), a short-acting estrogen, and testosterone enanthate benzilic acid hydrazone (TEBH), a long-acting androgen/anabolic steroid. EDE has not been made available for medical use alone. The medication, in combination with EB and TEBH, was given by injection into muscle once or at regular intervals, for instance once every 6 weeks.

Estradiol benzoate butyrate (EBB), sold under the brand names Neolutin N, Redimen, Soluna, and Unijab and formerly known under the developmental code name Unimens, is an estrogen medication which is used in hormonal birth control for women. It is formulated in combination with dihydroxyprogesterone acetophenide, a progestin, and is used specifically as a combined injectable contraceptive. EBB is not available for medical use alone. The medication, in combination with DHPA, is given by injection into muscle once a month.

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in feminizing hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

Estradiol benzoate/progesterone (EB/P4), sold under the brand names Duogynon and Sistocyclin among others, is a combination medication of estradiol benzoate (EB), an estrogen, and progesterone (P4), a progestogen. It has been formulated both as short-acting oil solutions and long-acting microcrystalline aqueous suspensions and is given by injection into muscle either once or continuously at regular intervals.

Estradiol benzoate/testosterone isobutyrate (EB/TiB), sold under the brand names Femandren M and Folivirin, is an injectable combination medication of estradiol benzoate (EB), an estrogen, and testosterone isobutyrate (TiB), an androgen/anabolic steroid, which is used in menopausal hormone therapy for women. It is provided in the form of 1 mL ampoules containing 2.5 mg estradiol benzoate and 25 mg testosterone isobutyrate in a microcrystalline aqueous suspension and is administered by intramuscular injection once every 4 to 6 weeks. EB/TiB reportedly has a duration of about 14 to 21 days.

Estradiol benzoate/estradiol dienanthate/testosterone enanthate benzilic acid hydrazone (EB/EDE/TEBH), sold under the brand names Climacteron, Lactimex, Lactostat, and Amenose, is an injectable combination medication of estradiol benzoate (EB), an estrogen, estradiol dienanthate (EDE), an estrogen, and testosterone enanthate benzilic acid hydrazone (TEBH), an androgen/anabolic steroid, which is used in menopausal hormone therapy for peri- and postmenopausal women and to suppress lactation in postpartum women. Clinical studies have assessed this formulation.
Estradiol benzoate/estradiol phenylpropionate/testosterone propionate/testosterone phenylpropionate/testosterone isocaproate (EB/EPP/TP/TPP/TiC), sold under the brand names Estandron Prolongatum, Lynandron Prolongatum, and Mixogen, was an injectable combination medication of the estrogens estradiol benzoate (EB) and estradiol phenylpropionate (EPP) and the androgens/anabolic steroids testosterone propionate (TP), testosterone phenylpropionate (TPP), and testosterone isocaproate (TiC) which was used in menopausal hormone therapy for women. It was also used to suppress lactation in postpartum women.

Estradiol/estradiol enanthate (E2/E2-EN) is an injectable combination formulation of estradiol (E2), a short-acting estrogen, and estradiol enanthate (E2-EN), a long-acting estrogen, which was developed by Boehringer around 1960 for potential medical use but was never marketed. It contained 1 mg E2 and 9 mg E2-EN in oil solution and was intended for administration by intramuscular injection.
Estradiol benzoate/estradiol valerate/norethisterone acetate/testosterone enanthate (EB/EV/NETA/TE), sold under the brand name Ablacton, is an injectable combination medication of estradiol benzoate (EB), an estrogen, estradiol valerate (EV), an estrogen, norethisterone acetate (NETA), a progestin, and testosterone enanthate (TE), an androgen/anabolic steroid, which has been used to suppress lactation in women. It contains 5 mg EB, 8 mg EV, 20 mg NETA, and 180 mg TE in oil solution and is provided in the form of ampoules. It is given as a single intramuscular injection following childbirth. The medication was manufactured by Schering and was previously marketed in Italy and Spain, but is no longer available.
Estradiol benzoate/progesterone/testosterone propionate (EB/P4/TP), sold under the brand names Lukestra, Steratrin, Trihormonal, and Trinestryl, is an injectable combination medication of estradiol benzoate (EB), an estrogen, progesterone (P4), a progestogen, and testosterone propionate (TP), an androgen/anabolic steroid. It contained 1 to 3 mg EB, 20 to 25 mg P4, and 25 mg TP, was provided in the form of ampoules, and was administered by intramuscular injection. The medication was introduced by 1949 and was marketed in the United States, the United Kingdom, and Germany among other places. It is no longer available.













