
Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.

Cortisone acetate is a synthetic glucocorticoid corticosteroid and corticosteroid ester which is marketed in many countries throughout the world, including in the United States, the United Kingdom, and various other European countries. It is the C21 acetate ester of cortisone, and acts as a prodrug of cortisone in the body.

Estropipate, also known as piperazine estrone sulfate and sold under the brand names Harmogen, Improvera, Ogen, Ortho-Est, and Sulestrex among others, is an estrogen medication which is used mainly in menopausal hormone therapy in the treatment of menopausal symptoms. It is a salt of estrone sulfate and piperazine, and is transformed into estrone and estradiol in the body. It is taken by mouth.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Cloxestradiol acetate, also known as 17-(2,2,2-trichloroethoxy)estradiol O,O-diacetate, is a synthetic steroidal estrogen derived from estradiol. It is the O,O-diacetate ester of cloxestradiol, which, in contrast to cloxestradiol acetate, was never marketed.
Estradiol stearate (E2-17-St), also known as estradiol octadecanoate and sold under the brand name Depofollan, is a naturally occurring estrogen and an estrogen ester – specifically, the C17β stearate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound is one of the components that collectively constitute lipoidal estradiol, another of which is estradiol palmitate. It is extremely lipophilic and hydrophobic. Estradiol stearate has no affinity for the estrogen receptor, requiring transformation into estradiol via esterases for its estrogenic activity. The compound does not bind to sex hormone-binding globulin or α-fetoprotein, instead being transported by lipoproteins such as high-density lipoprotein and low-density lipoprotein.

Estradiol pivalate, also known as estradiol trimethyl acetate (E2-TMA) and sold under the brand name Estrotate, is an estrogen medication and an estrogen ester; specifically, a pivalic acid ester of estradiol. Literature sources are conflicting as to whether the ester is located at the C3 position or at the C17β position. It was marketed as an oil solution for intramuscular injection in the 1940s and 1950s. A combination of estradiol pivalate (1 mg/mL) and progesterone (10 mg/mL) in oil solution for intramuscular injection was available in 1949. An aqueous suspension of estradiol pivalate was also developed by 1950 although whether it was ever marketed is unclear.

Estriol acetate benzoate (JAN), or oestriol diacetate benzoate (BAN), is an estrogen medication. It is an estrogen ester, specifically, an ester of estriol.

Estrone tetraacetylglucoside is a semisynthetic, steroidal estrogen. It is an estrogen ester, specifically, an ester of estrone. The drug was marketed since at least 1942.

Hydroxyestrone diacetate, or 16α-hydroxyestrone diacetate, also known as 3,16α-dihydroxyestra-1,3,5(10)-trien-17-one 3,16α-diacetate, is a synthetic, steroidal estrogen which has been marketed in France, Spain, Brazil, and Argentina. It is a derivative of 16α-hydroxyestrone with an acetate esters attached at the C3 and C16α positions.

Estrone cyanate, or estrone 3-O-cyanate, also known as estrocyanate, is an estrogen and an estrogen ester – specifically, the 3-O-cyanate ester of estrone – which was investigated for potential use in birth control pills but was found to be of relatively low potency and ultimately was never marketed.
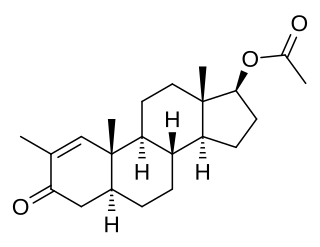
Stenbolone acetate (USAN), also known as 2-methyl-4,5α-dihydro-δ1-testosterone 17β-acetate or as 2-methyl-5α-androst-1-en-17β-ol-3-one 17β-acetate, is a synthetic, injected anabolic–androgenic steroid (AAS) and derivative of dihydrotestosterone (DHT) which has been marketed in Spain.

Metenolone acetate, or methenolone acetate, sold under the brand names Primobolan and Nibal, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of anemia due to bone marrow failure. It is taken by mouth. Although it was widely used in the past, the drug has mostly been discontinued and hence is now mostly no longer available. A related drug, metenolone enanthate, is given by injection into muscle.

Clostebol acetate (BAN), also known as 4-chlorotestosterone 17β-acetate (4-CLTA) or as 4-chloroandrost-4-en-17β-ol-3-one 17β-acetate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a derivative of testosterone that is marketed in Germany and Italy. It is an androgen ester – specifically, the C17β acetate ester of clostebol (4-chlorotestosterone) – and acts as a prodrug of clostebol in the body. Clostebol acetate is administered via intramuscular injection.

Estramustine is an estrogen and cytostatic antineoplastic agent which was never marketed. It is an estrogen ester – specifically, the C3 normustine ester of estradiol – and acts in part as a prodrug of estradiol in the body. Estramustine phosphate, the C17β phosphate ester of estramustine and a prodrug of estramustine, estromustine, estradiol, and estrone, is marketed and used in the treatment of prostate cancer.

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.

Estrone benzoate, or estrone 3-benzoate, is a synthetic estrogen and estrogen ester – specifically, the C3 benzoate ester of estrone – which was first reported in 1932 and was never marketed. It led to the development in 1933 of the more active estradiol benzoate, the first estradiol ester to be introduced for medical use.

Estrone methyl ether, or estrone 3-methyl ether, is a synthetic estrogen and estrogen ether – specifically, the C3 methyl ether of estrone – which was never marketed. It has been used to synthesize mestranol.
















