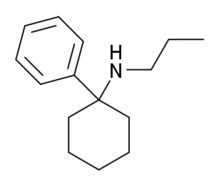
1-(1-Phenylcyclohexyl)-4-hydroxypiperidine (PCHP) is a metabolite of phencyclidine (PCP). PCHP can be detected in the hair, urine, stool, sweat, and saliva of PCP users.

PCAA, or 5-[N-(1-phenylcyclohexyl)]-aminopentanoic acid, is a metabolite of phencyclidine (PCP). It can be detected in the urine of PCP users by mass spectrometry as means of drug screening.

2C-T-2 is a psychedelic and entactogenic phenethylamine of the 2C family. It was first synthesized in 1981 by Alexander Shulgin, and rated by him as one of the "magical half-dozen" most important psychedelic phenethylamine compounds. The drug has structural and pharmacodynamic properties similar to those of 2C-T-7.

Ethylone, also known as 3,4-methylenedioxy-N-ethylcathinone, is a recreational designer drug classified as an entactogen, stimulant, and psychedelic of the phenethylamine, amphetamine, and cathinone chemical classes. It is the β-keto analogue of MDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone. In the United States, it began to be found in cathinone products in late 2011.

α-Pyrrolidinopropiophenone (α-PPP), is a stimulant drug. It is similar in structure to the appetite suppressant diethylpropion and has analogous effects in animals. Little is known about this compound, but it has been detected by laboratories in Germany as an ingredient in "ecstasy" tablets seized by law enforcement authorities. This drug has been found to produce stimulant effects in animals and presumably also produces these effects in humans, based on the context in which it has been found.

para-Methoxyphenylpiperazine is a piperazine derivative with stimulant effects which has been sold as an ingredient in "Party pills", initially in New Zealand and subsequently in other countries around the world.

Phthalimidopropiophenone is a chemical intermediate used in the synthesis of cathinone. It has been found to be sold on the illicit market as a controlled substance analogue, but little is currently known about its pharmacology or toxicology.
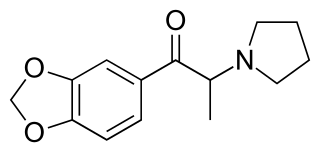
3',4'-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) is a stimulant designer drug. It was sold in Germany in the late 1990s and early 2000s as an ingredient in imitation ecstasy (MDMA) pills. It shares a similar chemical structure with α-PPP and MDPV, and has been shown to have reinforcing effects in rats.

4'-Methyl-α-pyrrolidinopropiophenone is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s, along with a number of other pyrrolidinophenone derivatives. Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends such as "NRG-3".

4'-Methoxy-α-pyrrolidinopropiophenone (MOPPP) is a stimulant designer drug of the pyrrolidinophenone class. It has the potential to produce euphoria, an effect shared with other classical stimulants.

4-Methylmethamphetamine (4-MMA) is a putative stimulant and entactogen drug of the amphetamine class. It is the β-deketo analogue of mephedrone.

3',4'-Methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP) is a stimulant of the cathinone class developed in the 1960s, which has been reported as a novel designer drug. MDPBP is sometimes sold under the name "NRG-1" as a mixture with other cathinone derivatives, including flephedrone, pentylone, MαPPP and its higher homologue MDPV. As with other cathinones, MDPBP has been shown to have reinforcing effects in rats.

α-Pyrrolidinopentiophenone is a synthetic stimulant of the cathinone class developed in the 1960s that has been sold as a designer drug. Colloquially, it is sometimes called flakka. α-PVP is chemically related to pyrovalerone and is the ketone analog of prolintane.

4'-Methyl-α-pyrrolidinobutiophenone or MPBP is a stimulant compound which has been reported as a novel designer drug. It is closely related to pyrovalerone, being simply its chain-shortened homologue.

4'-Methyl-α-pyrrolidinohexiophenone or MPHP is a stimulant compound which has been reported as a novel designer drug. It is closely related to pyrovalerone, being simply its chain-lengthened homologue. In the pyrrolidinophenone series, stimulant activity is maintained so long as the positions of the aryl, ketone and pyrrolidinyl groups are held constant, while the alkyl backbone can be varied anywhere between three and as many as seven carbons, with highest potency usually seen with the pentyl or isohexyl backbone, and a variety of substituents are tolerated on the aromatic ring.
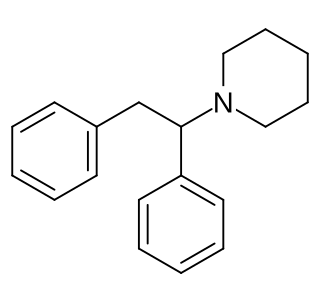
Diphenidine is a dissociative anesthetic that has been sold as a designer drug. The synthesis of diphenidine was first reported in 1924, and employed a Bruylants reaction analogous to the one that would later be used to discover phencyclidine in 1956. Shortly after the 2013 UK ban on arylcyclohexylamines, diphenidine and the related compound methoxphenidine became available on the grey market. Anecdotal reports describe high doses of diphenidine producing "bizarre somatosensory phenomena and transient anterograde amnesia." Diphenidine and related diarylethylamines have been studied in vitro as treatments for neurotoxic injury and are antagonists of the NMDA receptor. In dogs diphenidine exhibits greater antitussive potency than codeine phosphate.

3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative hallucinogen of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug.

4-Methylcathinone, is a stimulant drug of the cathinone chemical class that has been sold online as a designer drug. It is a metabolite of the better known drug mephedrone (4-methylmethcathinone).
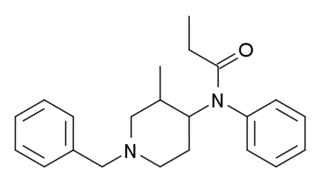
Isofentanyl (3-Methyl-benzylfentanyl) is an opioid analgesic that is an analog of fentanyl first invented in 1973, and which has been sold as a designer drug.