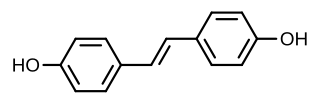
Stilbestrol, or stilboestrol, also known as 4,4'-dihydroxystilbene or 4,4'-stilbenediol, is a stilbenoid nonsteroidal estrogen and the parent compound of a group of more potent nonsteroidal estrogen derivatives that includes, most notably, diethylstilbestrol (DES). The term "stilbestrol" is often used incorrectly to refer to DES, but they are not the same compound.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.

Benzestrol is a synthetic nonsteroidal estrogen of the stilbestrol group which was formerly used medically but has since been discontinued. The stilbestrol estrogens, the best-known of which is diethylstilbestrol (DES) were used extensively in the mid-1900s and were finally banned by the FDA due to them causing tumors in the children of women who used them.

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.
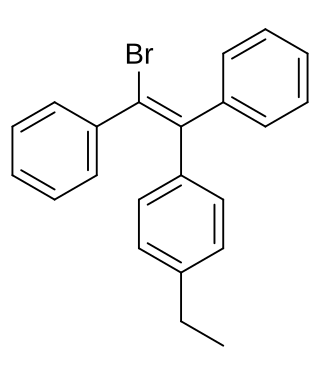
Broparestrol, also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active, and are similarly antiestrogenic but, unlike broparestrol, were never marketed.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Doisynoestrol, also known as fenocycline, as well as cis-bisdehydrodoisynolic acid 7-methyl ether, is a synthetic nonsteroidal estrogen of the doisynolic acid group that is no longer marketed. It is a methyl ether of bisdehydrodoisynolic acid. Doisynoestrol was described in the literature in 1945. It has about 0.02% of the relative binding affinity of estradiol for the estrogen receptor.
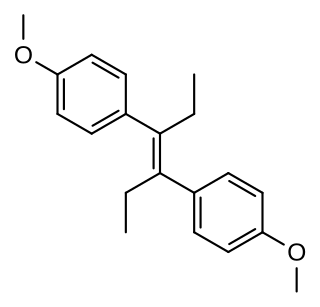
Dimestrol, also known as dianisylhexene, 4,4'-dimethoxy-α,α'-diethylstilbene, diethylstilbestrol dimethyl ether, and dimethoxydiethylstilbestrol, is a synthetic nonsteroidal estrogen of the stilbestrol group which is related to diethylstilbestrol. It has been used clinically as a hormonal therapy in cases of delayed female puberty, hypogonadism, menopausal, and postmenopausal symptoms. It is known to induce the development of female secondary sexual characteristics in the case of female delayed puberty or hypogonadism. The drug has also been used as a growth promoter in livestock.

Furostilbestrol (INN), also known as diethylstilbestrol di(2-furoate) or simply as diethylstilbestrol difuroate, is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol, that was never marketed. It is an ester of diethylstilbestrol and was described in the literature in 1952.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.

Dianol is a synthetic, nonsteroidal estrogen that was never marketed. It is a dimer and impurity of anol, and was, along with hexestrol, involved in erroneous findings of highly potent estrogenic activity with anol. Although a potent estrogen, it requires a dose of 100 μg to show activity, whereas hexestrol shows activity with a mere dose of 0.2 μg.

Mestilbol, also known as diethylstilbestrol monomethyl ether, is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It was developed by Wallace & Tiernan Company, patented in 1940, and introduced for medical use in the 1940s, but is now no longer marketed. Mestilbol was available both as oral tablets and in oil for intramuscular injection. The drug is gradually demethylated in the body into diethylstilbestrol and hence is a prodrug of diethylstilbestrol. Mestilbol is a highly active estrogen, although somewhat less so than diethylstilbestrol, but is longer-lasting in comparison.
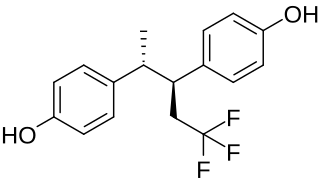
Terfluranol is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that was developed for the treatment of breast cancer but was never marketed. It was described in the medical literature in 1974.
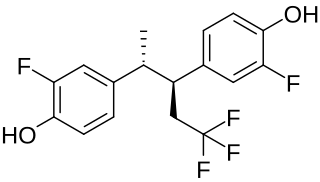
Pentafluranol is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that was developed for the treatment of benign prostatic hyperplasia never marketed. It was described in the medical literature in 1974.
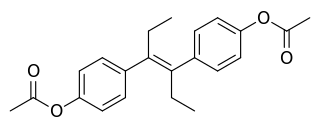
Diethylstilbestrol diacetate (DESDA) is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ester of diethylstilbestrol (DES) that was introduced for clinical use in the 1940s and was formerly marketed but is now no longer available.

Diethylstilbestrol disulfate is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ester of diethylstilbestrol (DES) that was formerly marketed but is now no longer available. It is described as an antineoplastic agent.
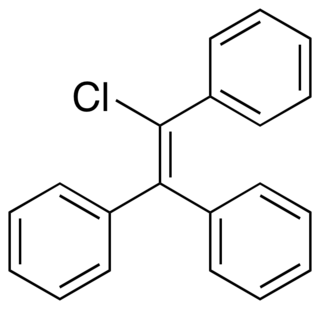
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.



















