
Chlorpromazine (CPZ), marketed under the brand names Thorazine and Largactil among others, is an antipsychotic medication. It is primarily used to treat psychotic disorders such as schizophrenia. Other uses include the treatment of bipolar disorder, severe behavioral problems in children including those with attention deficit hyperactivity disorder, nausea and vomiting, anxiety before surgery, and hiccups that do not improve following other measures. It can be given orally, by intramuscular injection, or intravenously.

Sepsis is a potentially life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs.

Septic shock is a potentially fatal medical condition that occurs when sepsis, which is organ injury or damage in response to infection, leads to dangerously low blood pressure and abnormalities in cellular metabolism. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defines septic shock as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by requiring a vasopressor to maintain a mean arterial pressure of 65 mm Hg or greater and having serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. This combination is associated with hospital mortality rates greater than 40%.

Clonidine, sold under the brand name Catapres among others, is an α2A-adrenergic receptor agonist medication used to treat high blood pressure, ADHD, drug withdrawal, menopausal flushing, diarrhea, spasticity, and certain pain conditions. The drug is often prescribed off-label for tics. It is used orally, by injection, or as a transdermal skin patch. Onset of action is typically within an hour with the effects on blood pressure lasting for up to eight hours.
Sympathomimetic drugs are stimulant compounds which mimic the effects of endogenous agonists of the sympathetic nervous system. Examples of sympathomimetic effects include increases in heart rate, force of cardiac contraction, and blood pressure. The primary endogenous agonists of the sympathetic nervous system are the catecholamines, which function as both neurotransmitters and hormones. Sympathomimetic drugs are used to treat cardiac arrest and low blood pressure, or even delay premature labor, among other things.

Phenylephrine, sold under the brand names Neosynephrine and Sudafed PE among others, is a medication used as a decongestant for uncomplicated nasal congestion in the form of a nasal spray or oral tablet, to dilate the pupil, to increase blood pressure given intravenously in cases of low blood pressure, and to relieve hemorrhoids as a suppository. It can also be applied to the skin.

Synephrine, or, more specifically, p-synephrine, is an alkaloid, occurring naturally in some plants and animals, and also in approved drugs products as its m-substituted analog known as neo-synephrine. p-Synephrine and m-synephrine are known for their longer acting adrenergic effects compared to epinephrine and norepinephrine. This substance is present at very low concentrations in common foodstuffs such as orange juice and other orange products, both of the "sweet" and "bitter" variety. The preparations used in traditional Chinese medicine (TCM), also known as Zhi Shi (枳实), are the immature and dried whole oranges from Citrus aurantium. Extracts of the same material or purified synephrine are also marketed in the US, sometimes in combination with caffeine, as a weight-loss-promoting dietary supplement for oral consumption. While the traditional preparations have been in use for millennia as a component of TCM-formulas, synephrine itself is not an approved over the counter drug. As a pharmaceutical, m-synephrine (phenylephrine) is still used as a sympathomimetic, mostly by injection for the treatment of emergencies such as shock, and rarely orally for the treatment of bronchial problems associated with asthma and hay-fever.
Alpha-1 blockers constitute a variety of drugs that block the effect of catecholamines on alpha-1-adrenergic receptors. They are mainly used to treat benign prostatic hyperplasia (BPH), hypertension and post-traumatic stress disorder. Alpha-1-adrenergic receptors are present in vascular smooth muscle, the central nervous system, and other tissues. When alpha blockers bind to these receptors in vascular smooth muscle, they cause vasodilation.
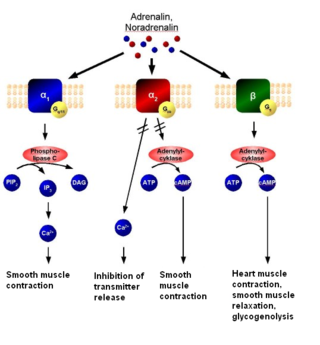
The alpha-2 (α2) adrenergic receptor is a G protein-coupled receptor (GPCR) associated with the Gi heterotrimeric G-protein. It consists of three highly homologous subtypes, including α2A-, α2B-, and α2C-adrenergic. Some species other than humans express a fourth α2D-adrenergic receptor as well. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α2-adrenergic receptor in the central and peripheral nervous systems.

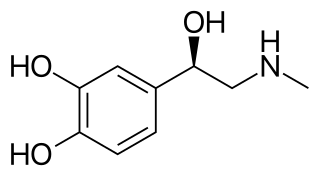
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic.

Dopexamine is a synthetic analogue of dopamine that is administered intravenously in hospitals to reduce exacerbations of heart failure and to treat heart failure following cardiac surgery. It is not used often, as more established drugs like epinephrine, dopamine, dobutamine, norepinephrine, and levosimendan work as well. It works by stimulating beta-2 adrenergic receptors and peripheral dopamine receptor D1 and dopamine receptor D2. It also inhibits the neuronal re-uptake of norepinephrine.

Benzoctamine is a drug that possesses sedative and anxiolytic properties. Marketed as Tacitin by Ciba-Geigy, it is different from most sedative drugs because in most clinical trials it does not produce respiratory depression, but actually stimulates the respiratory system. As a result, when compared to other sedative and anxiolytic drugs such as benzodiazepines like diazepam, it is a safer form of tranquilizing. However, when co-administered with other drugs that cause respiratory depression, like morphine, it can cause increased respiratory depression.

Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions. It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata. It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node, pupil dilation response, and blood sugar level. It does this by binding to alpha and beta receptors. It is found in many animals, including humans, and some single-celled organisms. It has also been isolated from the plant Scoparia dulcis found in Northern Vietnam.
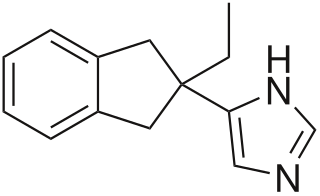
Atipamezole, sold under the brand name Antisedan among others, is a synthetic α2 adrenergic receptor antagonist used for the reversal of the sedative and analgesic effects of dexmedetomidine and medetomidine in dogs. Its reversal effect works by competing with the sedative for α2-adrenergic receptors and displacing them. It is mainly used in veterinary medicine, and while it is only licensed for dogs and for intramuscular use, it has been used intravenously, as well as in cats and other animals(intravenous use in cats and dogs is not recommended due to the potential for cardiovascular collapse. This occurs due to profound hypotension caused by reversal of the alpha 1 effects while the reflex bradycardia is still in effect.). There is a low rate of side effects, largely due to atipamezole's high specificity for the α2-adrenergic receptor. Atipamezole has a very quick onset, usually waking an animal up within 5 to 10 minutes.

Alpha blockers, also known as α-blockers or α-adrenoreceptor antagonists, are a class of pharmacological agents that act as antagonists on α-adrenergic receptors (α-adrenoceptors).

Norepinephrine, also known as noradrenaline and sold under the brand name Levophed among others, is a medication used to treat people with very low blood pressure. It is the typical medication used in sepsis if low blood pressure does not improve following intravenous fluids. It is the same molecule as the hormone and neurotransmitter norepinephrine. It is given by slow injection into a vein.

Epinephrine, also known as adrenaline, is a medication and hormone. As a medication, it is used to treat several conditions, including anaphylaxis, cardiac arrest, asthma, and superficial bleeding. Inhaled epinephrine may be used to improve the symptoms of croup. It may also be used for asthma when other treatments are not effective. It is given intravenously, by injection into a muscle, by inhalation, or by injection just under the skin.

Vasopressin infusions are in use for septic shock patients not responding to fluid resuscitation or infusions of catecholamines to increase the blood pressure while sparing the use of catecholamines. These argipressins have much shorter elimination half-life than synthetic non-arginine vasopresines with much longer elimination half-life of many hours. Further, argipressins act on V1a, V1b, and V2 receptors which consequently lead to higher eGFR and lower vascular resistance in the lungs. A number of injectable arginine vasopressins are in clinical use in the United States and the European Union. Pitressin among others, is a medication most commonly used in the treatment of frequent urination, increased thirst, and dehydration such as that resulting from diabetes insipidus, which causes increased and diluted urine. It is used to treat abdominal distension following some surgeries, and in stomach roentgenography. Vasopressin is a hormone that affects the kidneys and reduces urine flow.

Angiotensin II is a medication that is used to treat hypotension resulting from septic shock or other distributive shock. It is a synthetic vasoconstrictor peptide that is identical to human hormone angiotensin II and is marketed under the brand name Giapreza. The Food and Drug Administration approved the use of angiotensin II in December 2017 to treat low blood pressure resulting from septic shock.
Vasodilatory shock, vasogenic shock, or vasoplegic shock is a medical emergency belonging to shock along with cardiogenic shock, septic shock, allergen-induced shock and hypovolemic shock. When the blood vessels suddenly relax, it results in vasodilation. In vasodilatory shock, the blood vessels are too relaxed leading to extreme vasodilation and blood pressure drops and blood flow becomes very low. Without enough blood pressure, blood and oxygen will not be pushed to reach the body's organs. If vasodilatory shock lasts more than a few minutes, the lack of oxygen starts to damage the body's organs. Vasodilatory shock like other types of shock should be treated quickly, otherwise it can cause permanent organ damage or death as a result of multiple organ dysfunction.
















