Sodium voltage-gated channel alpha subunit 9 is a sodium ion channel that, in humans, is encoded by the SCN9A gene. It is usually expressed at high levels in two types of neurons: the nociceptive (pain) neurons at the dorsal root ganglion (DRG) and trigeminal ganglion; and sympathetic ganglion neurons, which are part of the autonomic (involuntary) nervous system.
Redafamdastat (INNTooltip International Nonproprietary Name; developmental code names JZP-150, PF-04457845) is an inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50Tooltip half-maximal inhibitory concentration of 7.2 nM, and both analgesic and anti-inflammatory effects in animal studies comparable to those of the cyclooxygenase inhibitor naproxen. It was being developed by Jazz Pharmaceuticals for the treatment of alcoholism, pain, and post-traumatic stress disorder (PTSD) and reached phase 2 clinical trials. However, development of the drug was discontinued in December 2023.

Funapide (INN) is a novel analgesic under development by Xenon Pharmaceuticals for the treatment of a variety of chronic pain conditions, including osteoarthritis, neuropathic pain, postherpetic neuralgia, and erythromelalgia, as well as dental pain. It acts as a small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker. Funapide is being evaluated in humans in both oral and topical formulations, and as of July 2014, has reached phase IIb clinical trials.
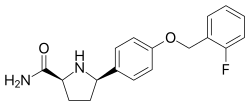
PF-05089771 is a selective, small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker under development by Pfizer as a novel analgesic. As of June 2014, it has completed phase II clinical trials for wisdom tooth removal and primary erythromelalgia.

DSP-2230 is a selective small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker which is under development by Dainippon Sumitomo Pharma for the treatment of neuropathic pain. As of June 2014, it is in phase I/phase II clinical trials.
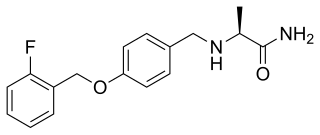
Ralfinamide (INN) is a multimodal drug which is under investigation by Newron Pharmaceuticals for the treatment of neuropathic pain and other pain conditions such as post-operative dental pain.
Evenamide is a selective voltage-gated sodium channel blocker, including subtypes Nav1.3, Nav1.7, and Nav1.8, which is described as an antipsychotic and is under development by Newron Pharmaceuticals as an add-on therapy for the treatment of schizophrenia. The drug has shown efficacy in animal models of psychosis, mania, depression, and aggression. It has completed phase I clinical trials, and phase II clinical trials will be commenced in the third quarter of 2015.

Cioteronel is a nonsteroidal antiandrogen (NSAA) that was never marketed. It was under development between 1989 and 2001 for the topical treatment of androgenetic alopecia, and acne and for the oral treatment of benign prostatic hyperplasia; it reached phase III clinical trials for acne and phase II studies for androgenetic alopecia, but was ultimately discontinued due to poor efficacy.

Erteberel is a synthetic, nonsteroidal estrogen which acts as a selective ERβ agonist and was under development by Eli Lilly for the treatment of schizophrenia. It was specifically under investigation for the treatment of negative symptoms and cognitive impairment associated with the condition. It managed to reach phase II clinical trials for this indication in the United States in 2015. As of 2021 development has been discontinued. Erteberel was also under investigation for the treatment of benign prostatic hyperplasia and reached phase II clinical studies for this use but failed to improve symptoms in men with the condition and development for this indication was discontinued. The drug has also been proposed as a potential novel treatment for glioblastoma.

Vilaprisan is a synthetic and steroidal selective progesterone receptor modulator (SPRM) which is under development by Bayer HealthCare Pharmaceuticals for the treatment of endometriosis and uterine fibroids. It is a potent and highly selective partial agonist of the progesterone receptor (PR). As of 2017, the drug is in phase II clinical trials for the aforementioned indications.

Pavinetant (INNTooltip International Nonproprietary Name, USANTooltip United States Adopted Name; developmental code names MLE-4901, AZD-4901, AZ-12472520, AZD-2624), is a small-molecule, orally active, selective neurokinin-3 (NK3) receptor antagonist which was under development by AstraZeneca and Millendo Therapeutics for the treatment of hot flashes and polycystic ovary syndrome (PCOS). It was also under investigation for the treatment of schizophrenia, but development was discontinued for this indication due to lack of effectiveness. In November 2017, development of the medication for hot flashes and PCOS was also terminated after its developer assessed the clinical risks and benefits.

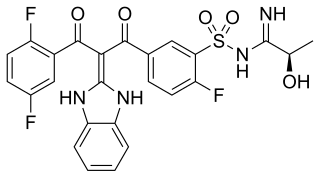
Opiranserin (INNTooltip International Nonproprietary Name; developmental code name VVZ-149) is a selective and combined glycine GlyT2 transporter blocker (IC50Tooltip Half-maximal inhibitory concentration = 0.86 μM), purine P2X3 receptor antagonist (IC50 = 0.87 μM), and serotonin 5-HT2A receptor antagonist (IC50Tooltip Half-maximal inhibitory concentration = 1.3 μM) which is under development by Vivozon for the intravenous treatment of postoperative pain. As of April 2017, it is in phase II clinical trials for this indication. The INNTooltip International Nonproprietary Name of the drug was issued in 2017. Approved for post-operative pain management in South Korea in December,2024.

Amesergide is a serotonin receptor antagonist of the ergoline and lysergamide families related to methysergide which was under development by Eli Lilly and Company for the treatment of a variety of conditions including depression, anxiety, schizophrenia, male sexual dysfunction, migraine, and thrombosis but was never marketed. It reached phase II clinical trials for the treatment of depression, erectile dysfunction, and premature ejaculation prior to the discontinuation of its development.
Opigolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist which was under development by Astellas Pharma for the treatment of endometriosis and rheumatoid arthritis. It was also under investigation for the treatment of prostate cancer. It reached phase II clinical trials for both endometriosis and rheumatoid arthritis prior to the discontinuation of its development in April 2018.

Ralmitaront is an investigational antipsychotic drug which is undergoing a clinical trial for the treatment of negative symptoms in schizophrenia and schizoaffective disorder. Another clinical trial targeting acute psychotic symptoms of schizophrenia has been terminated due to lack of efficacy. It is a partial agonist of the TAAR1. The medication is being developed by the pharmaceutical company Hoffmann-La Roche. Ralmitaront had completed phase 1 clinical trials.
N58A is a peptide depressant β-neurotoxin found in the venom of certain East Asian scorpions. The toxin affects voltage-gated sodium channels, specifically Nav1.8 & Nav1.9 channels.

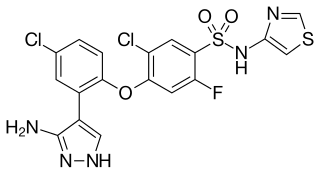
Suzetrigine is a non-opioid, small-molecule analgesic that works as a selective inhibitor of Nav1.8-dependent pain-signaling pathways in the peripheral nervous system. It is being developed by Vertex Pharmaceuticals and has completed two phase 3 clinical trials.

Ampreloxetine is a selective norepinephrine reuptake inhibitor (NRI) which is under development for the treatment of symptomatic neurogenic orthostatic hypotension (NOH).

Alogabat is an α5 subunit-containingGABAA receptor positive allosteric modulator which is under development for the treatment of pervasive developmental disorders and Angelman syndrome. It is taken by mouth.