
Ilmenite is a titanium-iron oxide mineral with the idealized formula FeTiO
3. It is a weakly magnetic black or steel-gray solid. Ilmenite is the most important ore of titanium and the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4", as a phonetic representation of the symbols of its molecular formula.

Chromium(III) fluoride is an inorganic compound with the chemical formula CrF3. It forms several hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H2O)6]F3 (violet) and [Cr(H2O)6]F3·3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.

Uranyl chloride refers to inorganic compounds with the formula UO2Cl2(H2O)n where n = 0, 1, or 3. These are yellow-colored salts.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Ammonium bifluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.
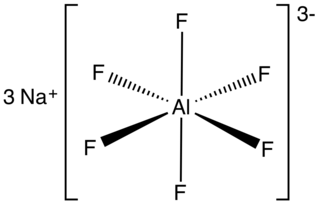
Sodium hexafluoroaluminate is an inorganic compound with formula Na3AlF6. This white solid, discovered in 1799 by Peder Christian Abildgaard (1740–1801), occurs naturally as the mineral cryolite and is used extensively in the industrial production of aluminium. The compound is the sodium (Na+) salt of the hexafluoroaluminate (AlF63−) ion.
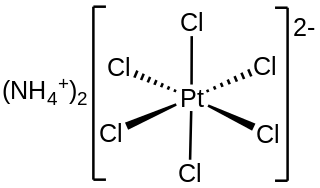
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2[PtCl6]. It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. In the presence of 1M NH4Cl, its solubility is only 0.0028 g/100 mL.

Ammonium paratungstate (or APT) is a white crystalline salt with the chemical formula (NH4)10(H2W12O42)·4H2O. It is described as "the most important raw material for all other tungsten products."
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.
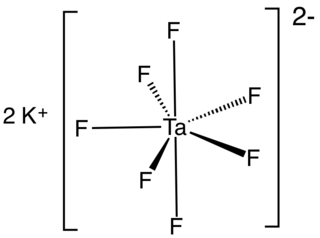
Potassium heptafluorotantalate is an inorganic compound with the formula K2[TaF7]. It is the potassium salt of the heptafluorotantalate anion [TaF7]2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.
Ammonium orthomolybdate is the inorganic compound with the chemical formula (NH4)2MoO4. It is a white solid that is prepared by treating molybdenum trioxide with aqueous ammonia. Upon heating these solutions, ammonia is lost, to give ammonium heptamolybdate ((NH4)6Mo7O24·4H2O).
![<span class="mw-page-title-main">Ammonium cyanate</span> Ionic chemical compound with formula [NH4]+ [OCN]-](https://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Ammonium-cyanate-3D-vdW.png/320px-Ammonium-cyanate-3D-vdW.png)
Ammoniumcyanate is an inorganic compound with the formula [NH4]+[OCN]−. It is a colorless, solid salt.

Ammonium hexachloroiridate(IV) is the inorganic compound with the formula (NH4)2[IrCl6]. This dark red solid is the ammonium salt of the iridium(IV) complex [IrCl6]2−. It is a commercially important iridium compound one of the most common complexes of iridium(IV). A related but ill-defined compound is iridium tetrachloride, which is often used interchangeably.

Ammonium tetrafluoroborate (or ammonium fluoroborate) is an inorganic salt composed of the ammonium cation and the tetrafluoroborate anion, with the chemical formula NH4BF4. When heated to decomposition, ammonium tetrafluoroborate releases toxic fumes of hydrogen fluoride, nitrogen oxides, and ammonia.
The +4 oxidation state dominates titanium chemistry, but compounds in the +3 oxidation state are also numerous. Commonly, titanium adopts an octahedral coordination geometry in its complexes, but tetrahedral TiCl4 is a notable exception. Because of its high oxidation state, titanium(IV) compounds exhibit a high degree of covalent bonding.

Hexafluorotitanic acid (systematically named oxonium hexafluoridotitanate(2-)) is an inorganic compound with the chemical formula (H3O)(H5O2)[TiF6]. According to X-ray crystallography, the salt consists of [TiF6]2- octahedral and two kinds of oxonium cations, (H3O)+ and (H5O2)+.
Lithium hexafluorotitanate is an inorganic compound of lithium, fluorine, and titanium with the chemical formula Li2TiF6.











![<span class="mw-page-title-main">Ammonium cyanate</span> Ionic chemical compound with formula [NH4]+ [OCN]-](https://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Ammonium-cyanate-3D-vdW.png/320px-Ammonium-cyanate-3D-vdW.png)


