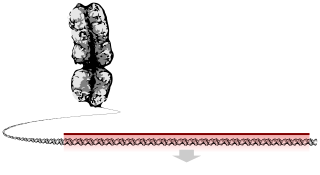
Glycophorin C plays a functionally important role in maintaining erythrocyte shape and regulating membrane material properties, possibly through its interaction with protein 4.1. Moreover, it has previously been shown that membranes deficient in protein 4.1 exhibit decreased content of glycophorin C. It is also an integral membrane protein of the erythrocyte and acts as the receptor for the Plasmodium falciparum protein PfEBP-2.

Duffy antigen/chemokine receptor (DARC), also known as Fy glycoprotein (FY) or CD234, is a protein that in humans is encoded by the DARC gene.

Complement receptor type 1 (CR1) also known as C3b/C4b receptor or CD35 is a protein that in humans is encoded by the CR1 gene.

CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.

The ABO blood group system is used to denote the presence of one, both, or neither of the A and B antigens on erythrocytes. In human blood transfusions it is the most important of the 36 different blood type classification systems currently recognized. A very rare mismatch in this, or any other serotype, can cause a serious, potentially fatal, adverse reaction after a transfusion, or a contra-indicated immune response to an organ transplant. The associated anti-A and anti-B antibodies are usually IgM antibodies, which are produced in the first years of life by sensitization to environmental substances, such as food, bacteria, and viruses.
The Kell antigen system is a group of antigens on the human red blood cell surface which are important determinants of blood type and are targets for autoimmune or alloimmune diseases which destroy red blood cells. Kell can be noted as K, k, or Kp. The Kell antigens are peptides found within the Kell protein, a 93-kilodalton transmembrane zinc-dependent endopeptidase which is responsible for cleaving endothelin-3.

McLeod syndrome is an X-linked recessive genetic disorder that may affect the blood, brain, peripheral nerves, muscle, and heart. It is caused by a variety of recessively inherited mutations in the XK gene on the X chromosome. The gene is responsible for producing the Kx protein, a secondary supportive protein for the Kell antigen on the red blood cell surface.
Animal erythrocytes have cell surface antigens that undergo polymorphism and give rise to blood types. Antigens from the human ABO blood group system are also found in apes and Old World monkeys, and the system traces back to the origin of hominoids. Other animal blood sometimes agglutinates with human blood group reagents, but the structure of the blood group antigens in animals is not always identical to those typically found in humans. The classification of most animal blood groups therefore uses different blood typing systems to those used for classification of human blood.
The MNS antigen system is a human blood group system based upon two genes on chromosome 4. There are currently 46 antigens in the system, but the five most important are called M, N, S, s, and U.
The LW blood system was first described by Landsteiner and Wiener in 1940. It was often confused with the Rh system, not becoming a separate antigen system until 1982. The LW and RhD antigens are genetically independent though they are phenotypically related and the LW antigen is expressed more strongly on RhD positive cells than on RhD negative cells. In most populations, the antithetical LW antigens, LWa and LWb are present as very high and very low frequency, respectively.

Galactoside 3(4)-L-fucosyltransferase is an enzyme that in humans is encoded by the FUT3 gene.

Blood group Rh(CE) polypeptide is a protein that in humans is encoded by the RHCE gene. RHCE has also recently been designated CD240CE.

Rh-associated glycoprotein (RHAG) is an ammonia transporter protein that in humans is encoded by the RHAG gene. RHAG has also recently been designated CD241. Mutations in the RHAG gene can cause stomatocytosis.

Ecto-ADP-ribosyltransferase 4 is an enzyme that in humans is encoded by the ART4 gene. ART4 has also been designated as CD297.

Erythroid membrane-associated protein is a protein that in humans is responsible for the Scianna blood group system, and is encoded by the ERMAP gene.

Glycophorin-E is a protein that in humans is encoded by the GYPE gene.
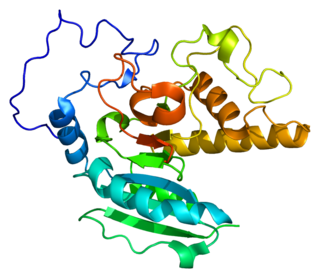
Histo-blood group ABO system transferase is an enzyme with glycosyltransferase activity, which is encoded by the ABO gene in humans. It is ubiquitously expressed in many tissues and cell types. ABO determines the ABO blood group of an individual by modifying the oligosaccharides on cell surface glycoproteins. Variations in the sequence of the protein between individuals determine the type of modification and the blood group. The ABO gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.
The BGMUT Database documents allelic variations in the genes encoding for human blood group systems. It was set up in 1999 through an initiative of the Human Genome Variation Society (HGVS). Since 2006, it has been a part of the dbRBC resource of NCBI at the NIH. In addition to being a repository of the genetic variations of the blood group antigen-encoding genes, the database also provides information on the blood group systems, the genes that encode them, the serological phenotypes associated with the alleles of the genes, etc. Information on genetic variations in some non-human orthologous genes is also provided.

Rh blood group, D antigen also known as Rh polypeptide 1 (RhPI) or cluster of differentiation 240D (CD240D) is a protein that in humans is encoded by the RHD gene.