Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as medications to decrease blood pressure in patients with hypertension. CCBs are particularly effective against large vessel stiffness, one of the common causes of elevated systolic blood pressure in elderly patients. Calcium channel blockers are also frequently used to alter heart rate, to prevent peripheral and cerebral vasospasm, and to reduce chest pain caused by angina pectoris.
A calcium channel is an ion channel which shows selective permeability to calcium ions. It is sometimes synonymous with voltage-gated calcium channel, although there are also ligand-gated calcium channels.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g., muscle, glial cells, neurons, etc.) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+-Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions. At physiologic or resting membrane potential, VGCCs are normally closed. They are activated (i.e., opened) at depolarized membrane potentials and this is the source of the "voltage-gated" epithet. The concentration of calcium (Ca2+ ions) is normally several thousand times higher outside the cell than inside. Activation of particular VGCCs allows Ca2+ to rush into the cell, which, depending on the cell type, results in activation of calcium-sensitive potassium channels, muscular contraction, excitation of neurons, up-regulation of gene expression, or release of hormones or neurotransmitters. VGCCs have been immunolocalized in the zona glomerulosa of normal and hyperplastic human adrenal, as well as in aldosterone-producing adenomas (APA), and in the latter T-type VGCCs correlated with plasma aldosterone levels of patients. Excessive activation of VGCCs is a major component of excitotoxicity, as severely elevated levels of intracellular calcium activates enzymes which, at high enough levels, can degrade essential cellular structures.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
T-type calcium channels are low voltage activated calcium channels that open during cell membrane depolarization. These channels aid in mediating calcium influx into cells after an action potential or depolarizing signal. The entry of calcium into various cells has many different physiological responses associated with it. Within cardiac muscle cell and smooth muscle cells voltage-gated calcium channel activation initiates contraction directly by allowing the cytosolic concentration to increase. Not only are T-type calcium channels known to be present within cardiac and smooth muscle, but also are present in many neuronal cells within the central nervous system. Different experimental studies within the 1970s allowed for the distinction of T-type calcium channels from the already well-known L-type calcium channels. The new T-type channels were much different from the L-type calcium channels due to their ability to be activated by more negative membrane potentials, had small single channel conductance, and also were unresponsive to calcium antagonist drugs that were present. These distinct calcium channels are generally located within the brain, peripheral nervous system, heart, smooth muscle, bone, and endocrine system.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channels initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.
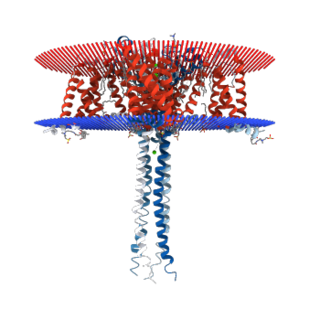
N-type calcium channels are voltage gated calcium channels that are distributed throughout the entire body. These channels are high voltage activated channels composed of alpha-1B subunits. The alpha subunit forms the pore through which the calcium enters and helps to determine most of the channel's properties. The alpha subunit is also known as the calcium channel/voltage dependent/N type, alpha 1 subunit (CACNA1B), or calcium voltage-gated channel subunit alpha1 B. The subunit is essential to modulate neurotransmitter release. They also contain associated subunits such as β1, β3, β4, α2δ, and possibly γ. These channels are known for their importance in the nervous system. They play a small role in the migration of immature neurons before the establishment of their mature synapses, and they are critically involved in the release of neurotransmitters, which is also similar to another type of calcium channels, known as P-type calcium channels. N-type calcium channels are targets for the development of drugs to relieve chronic and neuropathic pain. They are also used for the treatment of hypertension, Autism Spectrum Disorder, Osteoarthritis, and other medical diagnoses. Additionally, N-type calcium channels have known functions in the kidney, and heart. There are many known N-type calcium channel blockers that function to inhibit channel activity, although the most notable blockers are ω-Conotoxins. Blockers, like ω-Conotoxins, can interfere with many different biological and therapeutic processes.

The L-type calcium channel is part of the high-voltage activated family of voltage-dependent calcium channel.
"L" stands for long-lasting referring to the length of activation. This channel has four subunits.
Gq protein (Gαq, or Gq/11) is a heterotrimeric G protein subunit that activates phospholipase C (PLC). PLC in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to diacyl glycerol (DAG) and inositol trisphosphate (IP3) signal transduction pathway. DAG acts as a second messenger that activates protein kinase C (PKC) and IP3 helps in phosphorylation of some proteins.

The Cav2.1 P/Q voltage-dependent calcium channel is encoded by the CACNA1A gene.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.
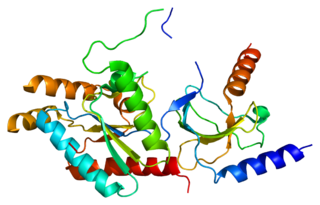
Voltage-dependent L-type calcium channel subunit beta-2 is a protein that in humans is encoded by the CACNB2 gene.

Voltage-dependent L-type calcium channel subunit beta-4 is a protein that in humans is encoded by the CACNB4 gene.

Voltage-dependent L-type calcium channel subunit beta-1 is a protein that in humans is encoded by the CACNB1 gene.

Calcium channel, voltage-dependent, gamma subunit 2, also known as CACNG2 or stargazin is a protein that in humans is encoded by the CACNG2 gene.

Voltage-dependent calcium channel subunit alpha-2/delta-1 is a protein that in humans is encoded by the CACNA2D1 gene.

Voltage-dependent calcium channel gamma-3 subunit is a protein that in humans is encoded by the CACNG3 gene.

Voltage-dependent calcium channel gamma-1 subunit is a protein that in humans is encoded by the CACNG1 gene.
Leconotide (INN), also known as ω-conotoxin CVID, is an ω-conotoxin peptide isolated from the venom of Conus catus which is under investigation as an analgesic drug for the treatment of pain conditions.











