Related Research Articles
In physiology, nociception, also nocioception; from Latin nocere 'to harm/hurt') is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal to trigger an appropriate defensive response.
A mechanoreceptor, also called mechanoceptor, is a sensory receptor that responds to mechanical pressure or distortion. Mechanoreceptors are innervated by sensory neurons that convert mechanical pressure into electrical signals that, in animals, are sent to the central nervous system.

A nociceptor is a sensory neuron that responds to damaging or potentially damaging stimuli by sending "possible threat" signals to the spinal cord and the brain. The brain creates the sensation of pain to direct attention to the body part, so the threat can be mitigated; this process is called nociception.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of taste, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.
Nanchung is an invertebrate TRP channel that acts to sense mechanical force. Drosophila nanchung mutants show deficits in antennal sensation, including hearing and hygrosensation, and are unable to transduce sound stimuli.
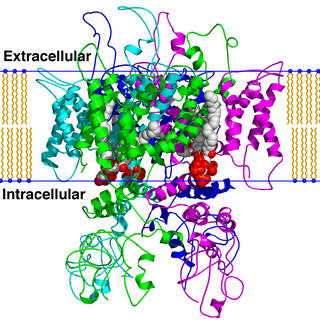
In cellular biology, mechanotransduction is any of various mechanisms by which cells convert mechanical stimulus into electrochemical activity. This form of sensory transduction is responsible for a number of senses and physiological processes in the body, including proprioception, touch, balance, and hearing. The basic mechanism of mechanotransduction involves converting mechanical signals into electrical or chemical signals.

Johnston's organ is a collection of sensory cells found in the pedicel of the antennae in the class Insecta. Johnston's organ detects motion in the flagellum. It consists of scolopidia arrayed in a bowl shape, each of which contains a mechanosensory chordotonal neuron. The number of scolopidia varies between species. In homopterans, the Johnston's organs contain 25 - 79 scolopidia. The presence of Johnston's organ is a defining characteristic which separates the class Insecta from the other hexapods belonging to the group Entognatha. Johnston's organ was named after the physician Christopher Johnston (1822-1891) father of the physician and Assyriologist Christopher Johnston.
Merkel nerve endings are mechanoreceptors, a type of sensory receptor, that are found in the basal epidermis and hair follicles. They are nerve endings and provide information on mechanical pressure, position, and deep static touch features, such as shapes and edges.

Campaniform sensilla are a class of mechanoreceptors found in insects, which respond to local stress and strain within the animal's cuticle. Campaniform sensilla function as proprioceptors that detect mechanical load as resistance to muscle contraction, similar to mammalian Golgi tendon organs. Sensory feedback from campaniform sensilla is integrated in the control of posture and locomotion.
Chordotonal organs are stretch receptor organs found only in insects and crustaceans. They are located at most joints and are made up of clusters of scolopidia that either directly or indirectly connect two joints and sense their movements relative to one another. They can have both extero- and proprioceptive functions, for example sensing auditory stimuli or leg movement. The word was coined by Vitus Graber in 1882, though he interpreted them as being stretched between two points like a string, sensing vibrations through resonance.
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.
Mechanosensation is the transduction of mechanical stimuli into neural signals. Mechanosensation provides the basis for the senses of light touch, hearing, proprioception, and pain. Mechanoreceptors found in the skin, called cutaneous mechanoreceptors, are responsible for the sense of touch. Tiny cells in the inner ear, called hair cells, are responsible for hearing and balance. States of neuropathic pain, such as hyperalgesia and allodynia, are also directly related to mechanosensation. A wide array of elements are involved in the process of mechanosensation, many of which are still not fully understood.

Proprioception is the sense of self-movement, force, and body position.
Mechanosensitive channels (MSCs), mechanosensitive ion channels or stretch-gated ion channels are membrane proteins capable of responding to mechanical stress over a wide dynamic range of external mechanical stimuli. They are present in the membranes of organisms from the three domains of life: bacteria, archaea, and eukarya. They are the sensors for a number of systems including the senses of touch, hearing and balance, as well as participating in cardiovascular regulation and osmotic homeostasis (e.g. thirst). The channels vary in selectivity for the permeating ions from nonselective between anions and cations in bacteria, to cation selective allowing passage Ca2+, K+ and Na+ in eukaryotes, and highly selective K+ channels in bacteria and eukaryotes.

A scolopidium is the fundamental unit of a mechanoreceptor organ in insects. It is a composition of three cells: a scolopale cap cell which caps the scolopale cell, and a bipolar sensory nerve cell.
Inactive is a TRPV channel in invertebrates. Inactive mutant flies show locomotor and hearing deficits.
Hair plates are a type of proprioceptor found in the folds of insect joints. They consist of a cluster of hairs, in which each hair is innervated by a single mechanosensory neuron. Functionally, hair plates operate as "limit-detectors" by signaling the extremes of joint movement, which then drives reflexive leg movement.

The femoral chordotonal organ is a group of mechanosensory neurons found in an insect leg that detects the movements and the position of the femur/tibia joint. It is thought to function as a proprioceptor that is critical for precise control of leg position by sending the information regarding the femur/tibia joint to the motor circuits in the ventral nerve cord and the brain
References
- ↑ Duggan, A.; García-Añoveros, J.; Corey, D. P. (2000). "Insect mechanoreception: What a long, strange TRP it's been". Current Biology. 10 (10): R384–R387. Bibcode:2000CBio...10.R384D. doi: 10.1016/s0960-9822(00)00478-4 . PMID 10837217.
- ↑ Walker, R. G.; Willingham, A. T.; Zuker, C. S. (2000). "A Drosophila mechanosensory transduction channel". Science. 287 (5461): 2229–2234. Bibcode:2000Sci...287.2229W. CiteSeerX 10.1.1.646.2497 . doi:10.1126/science.287.5461.2229. PMID 10744543.
- 1 2 Li, W.; Feng, Z.; Sternberg, P. W.; Shawn Xu, X. Z. (2006). "A C. Elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue". Nature. 440 (7084): 684–687. Bibcode:2006Natur.440..684L. doi:10.1038/nature04538. PMC 2865900 . PMID 16572173.
- 1 2 Shin, J. -B.; Adams, D.; Paukert, M.; Siba, M.; Sidi, S.; Levin, M.; Gillespie, P. G.; Gründer, S. (2005). "Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells". Proceedings of the National Academy of Sciences. 102 (35): 12572–12577. Bibcode:2005PNAS..10212572S. doi: 10.1073/pnas.0502403102 . PMC 1194908 . PMID 16116094.
- 1 2 Sidi, S.; Friedrich, R. W.; Nicolson, T. (2003). "NompC TRP Channel Required for Vertebrate Sensory Hair Cell Mechanotransduction". Science. 301 (5629): 96–99. Bibcode:2003Sci...301...96S. doi: 10.1126/science.1084370 . PMID 12805553. S2CID 23882972.
- ↑ Jin, Peng; Bulkley, David; Guo, Yanmeng; Zhang, Wei; Guo, Zhenhao; Huynh, Walter; Wu, Shenping; Meltzer, Shan; Cheng, Tong (July 2017). "Electron cryo-microscopy structure of the mechanotransduction channel NOMPC". Nature. 547 (7661): 118–122. Bibcode:2017Natur.547..118J. doi:10.1038/nature22981. ISSN 1476-4687. PMC 5669069 . PMID 28658211.
- 1 2 Howard, J.; Bechstedt, S. (2004). "Hypothesis: A helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors". Current Biology. 14 (6): R224–R226. Bibcode:2004CBio...14.R224H. doi: 10.1016/j.cub.2004.02.050 . PMID 15043829.
- ↑ Palmer, C. P.; Zhou, X. L.; Lin, J.; Loukin, S. H.; Kung, C.; Saimi, Y. (2001). "A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane". Proceedings of the National Academy of Sciences. 98 (14): 7801–7805. doi: 10.1073/pnas.141036198 . PMC 35422 . PMID 11427713.
- ↑ Cheng, L. E.; Song, W.; Looger, L. L.; Jan, L. Y.; Jan, Y. N. (2010). "The role of the TRP channel NompC in Drosophila larval and adult locomotion". Neuron. 67 (3): 373–380. doi:10.1016/j.neuron.2010.07.004. PMC 2933178 . PMID 20696376.
- ↑ Liang, X.; Madrid, J.; Saleh, H. S.; Howard, J. (2011). "NOMPC, a Member of the TRP Channel Family, Localizes to the Tubular Body and Distal Cilium of Drosophila Campaniform and Chordotonal Receptor Cells". Cytoskeleton. 68 (1): 1–7. doi:10.1002/cm.20493. PMC 3048163 . PMID 21069788.
- ↑ Lee, J.; Moon, S.; Cha, Y.; Chung, Y. D. (2010). Gonzalez, Cayetano (ed.). "Drosophila TRPN( =NOMPC) Channel Localizes to the Distal End of Mechanosensory Cilia". PLOS ONE. 5 (6): e11012. Bibcode:2010PLoSO...511012L. doi: 10.1371/journal.pone.0011012 . PMC 2882365 . PMID 20543979.
- ↑ Zhang, W; Yan, Z; Jan, L. Y.; Jan, Y. N. (2013). "Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae". Proceedings of the National Academy of Sciences. 110 (33): 13612–7. Bibcode:2013PNAS..11013612Z. doi: 10.1073/pnas.1312477110 . PMC 3746866 . PMID 23898199.
- 1 2 Lehnert, B. P.; Baker, A. E.; Gaudry, Q; Chiang, A. S.; Wilson, R. I. (2013). "Distinct roles of TRP channels in auditory transduction and amplification in Drosophila". Neuron. 77 (1): 115–28. doi:10.1016/j.neuron.2012.11.030. PMC 3811118 . PMID 23312520.
- ↑ Kang, L.; Gao, J.; Schafer, W. R.; Xie, Z.; Xu, X. Z. S. (2010). "C. Elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel". Neuron. 67 (3): 381–391. doi:10.1016/j.neuron.2010.06.032. PMC 2928144 . PMID 20696377.
- ↑ Schüler, Andreas; Schmitz, Gregor; Reft, Abigail; Özbek, Suat; Thurm, Ulrich; Bornberg-Bauer, Erich (June 2015). "The Rise and Fall of TRP-N, an Ancient Family of Mechanogated Ion Channels, in Metazoa". Genome Biology and Evolution. 7 (6): 1713–1727. doi: 10.1093/gbe/evv091 . PMC 4494053 . PMID 26100409.
- ↑ NCBI Genbank entry
- ↑ NCBI Genbank entry
- ↑ NCBI Genbank entry
- ↑ NCBI Genbank entry